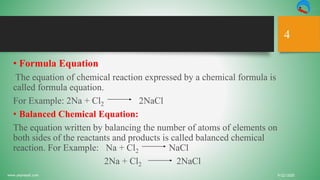
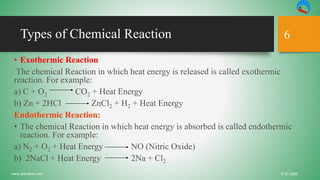
The document discusses chemical reactions, defining matter, physical and chemical changes, and presenting the types of chemical equations. It explains diatomic elements, balanced chemical equations, and the limitations of chemical equations. Additionally, it covers exothermic and endothermic reactions, as well as the roles of catalysts in chemical reactions.