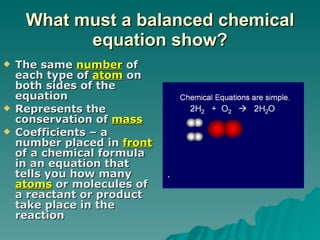
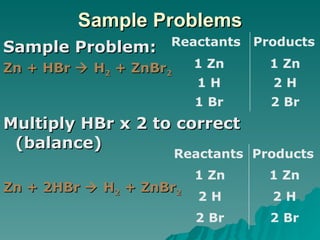
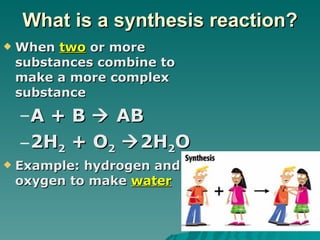
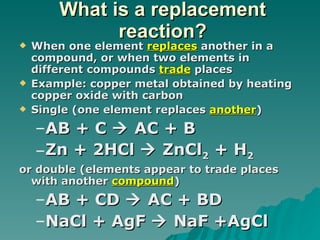
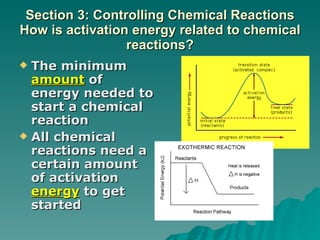
This document discusses chemical reactions and properties of matter. It defines physical and chemical properties, and explains that a chemical change produces new substances while a physical change does not. It also outlines how to identify chemical reactions through observation of properties like color change, gas production, or precipitate formation. The document then explains how to write and balance chemical equations, and categorizes three main types of chemical reactions: synthesis, decomposition, and replacement. Finally, it discusses factors that affect the rate of chemical reactions like surface area, temperature, concentration, and catalysts.