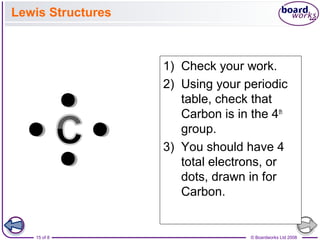
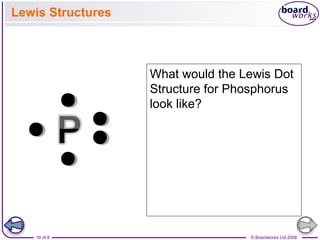
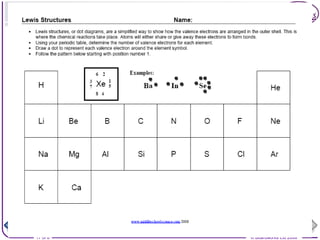
The document summarizes key information about atomic structure:
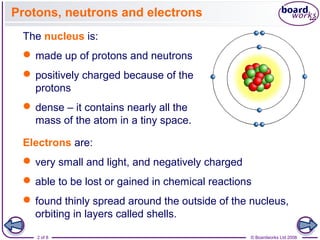
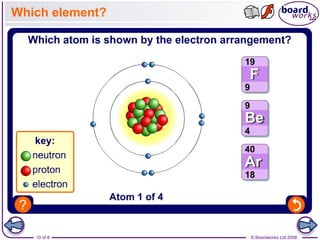
- The nucleus is positively charged and contains nearly all an atom's mass, while electrons are much smaller and negatively charged, orbiting in shells outside the nucleus.
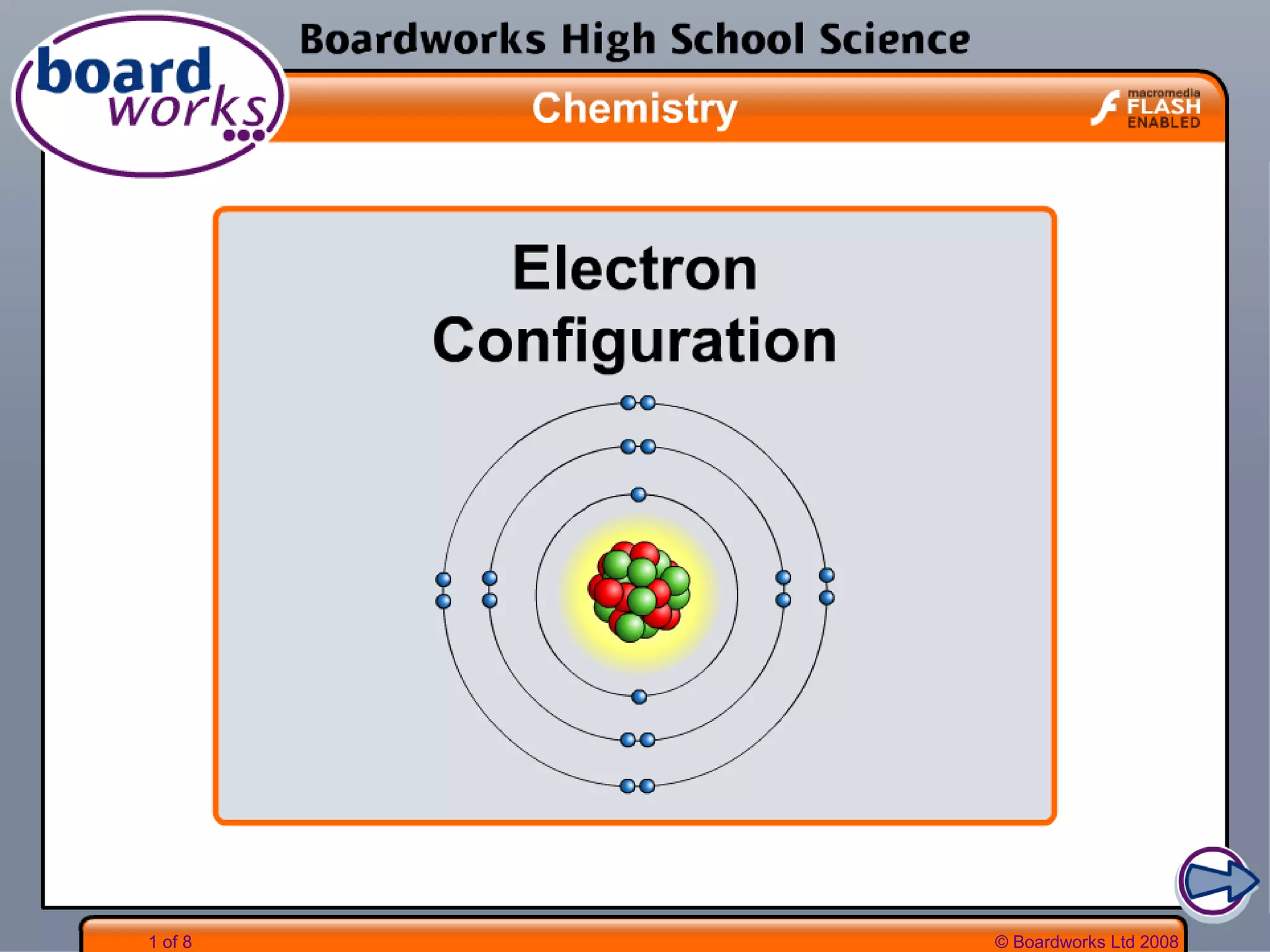
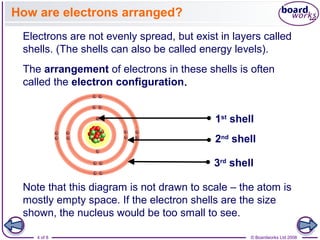
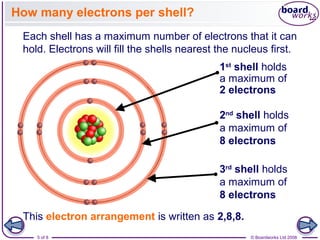
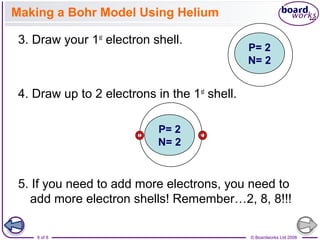
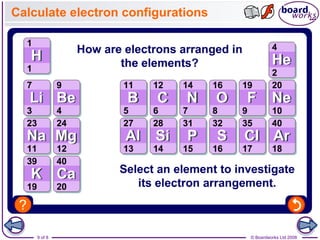
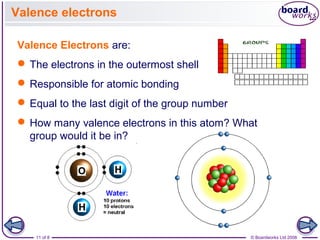
- Electrons are arranged in shells (also called energy levels) around the nucleus, with the first shell holding up to 2 electrons and subsequent shells holding up to 8 electrons each.
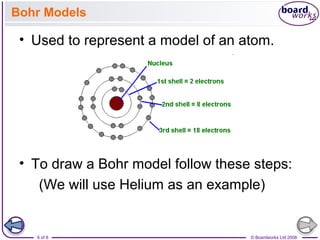
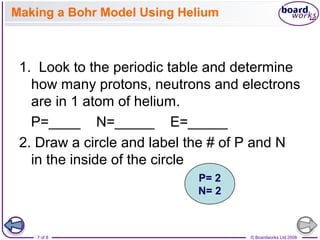
- Atoms can be represented using Bohr models that show the nucleus and electrons arranged in shells, with the number of protons and neutrons indicated in the nucleus.