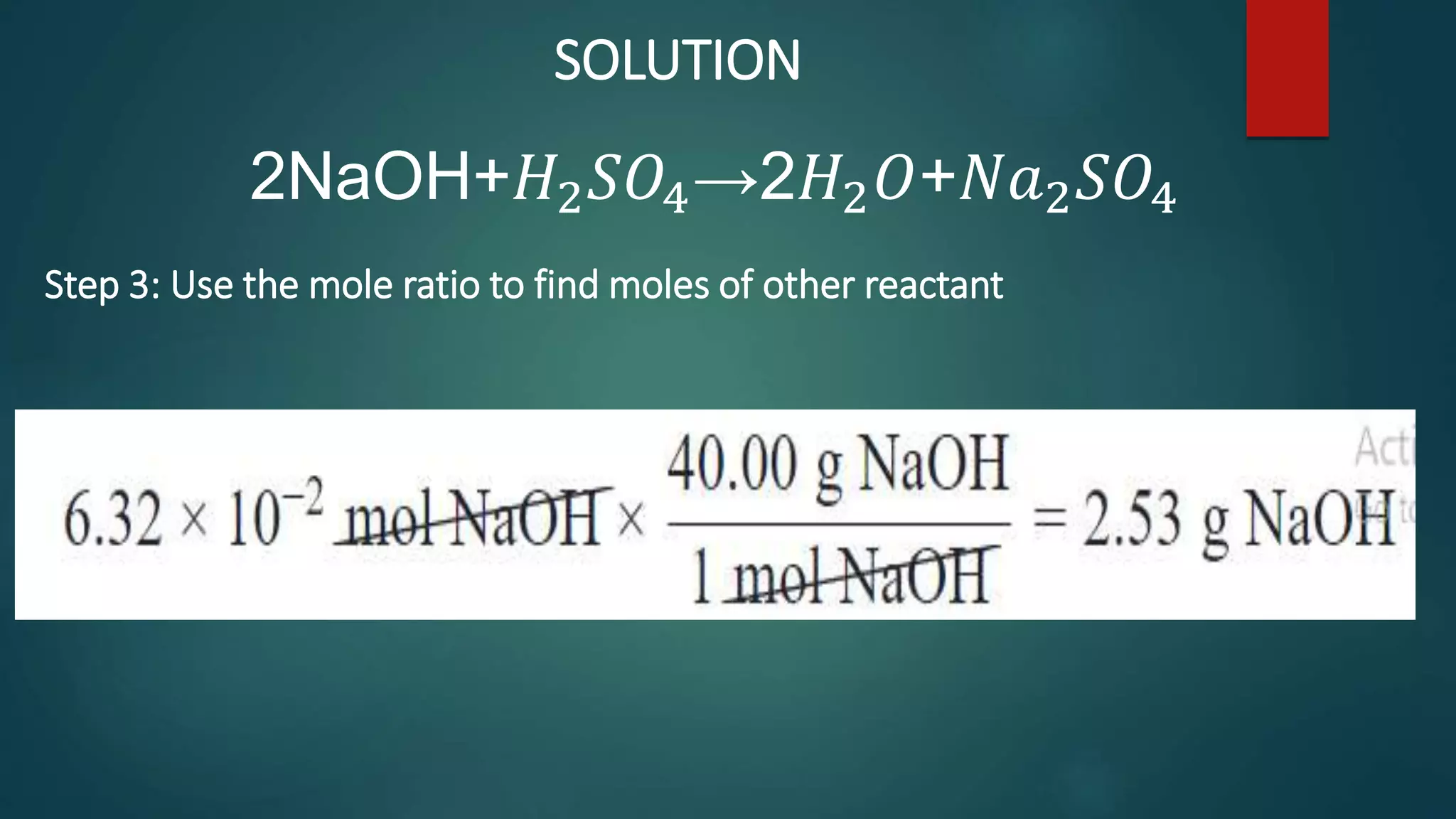
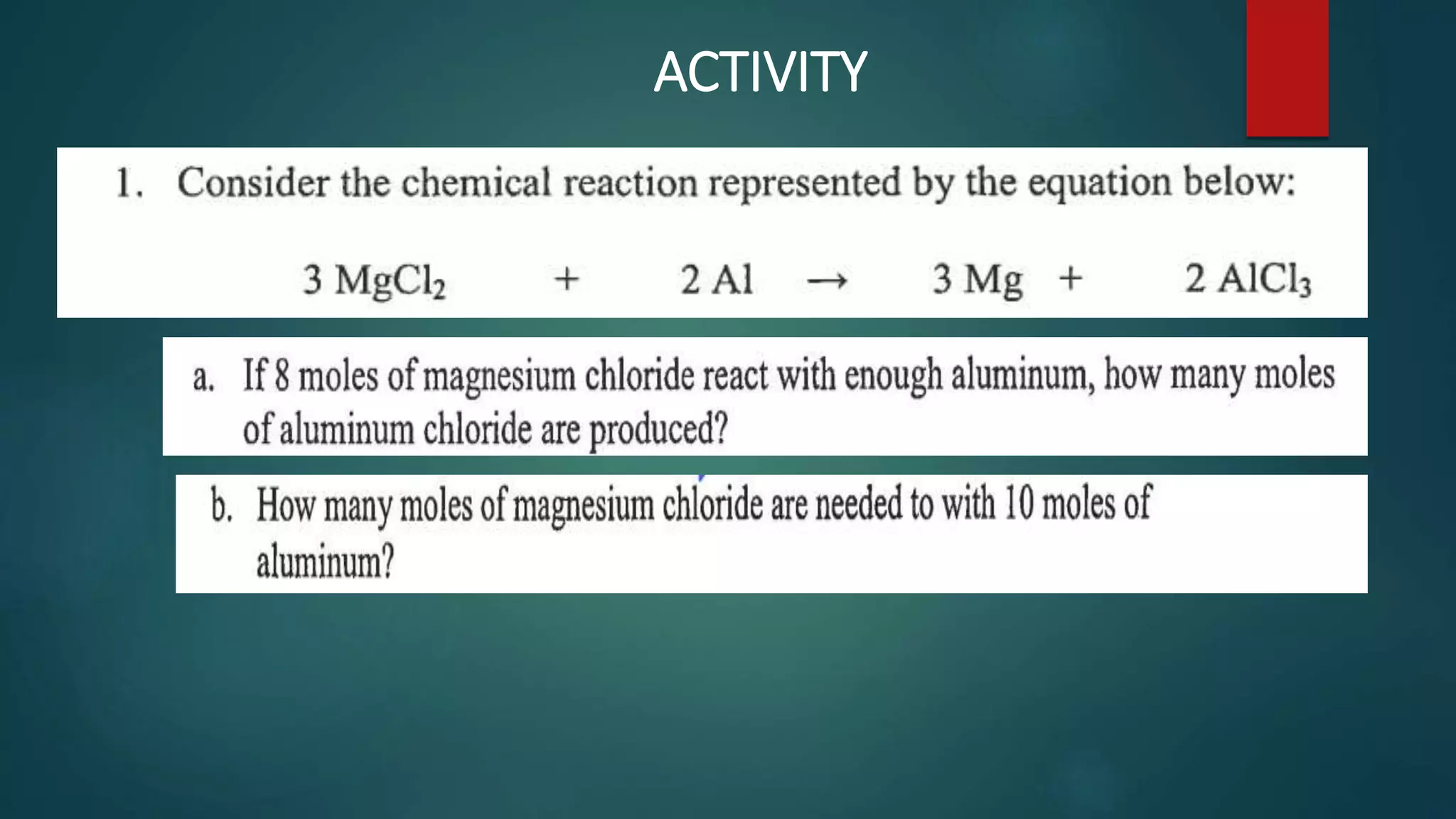
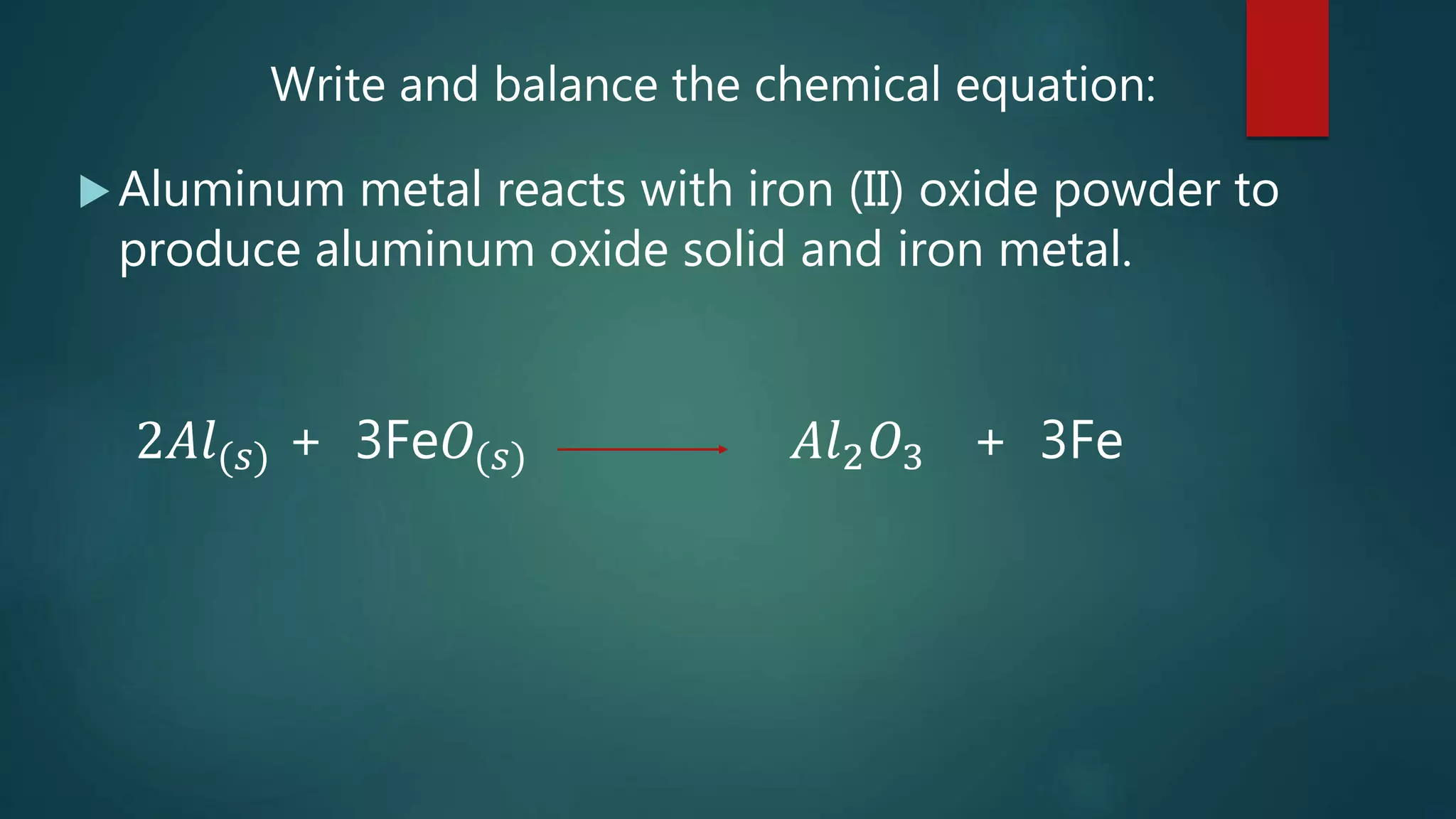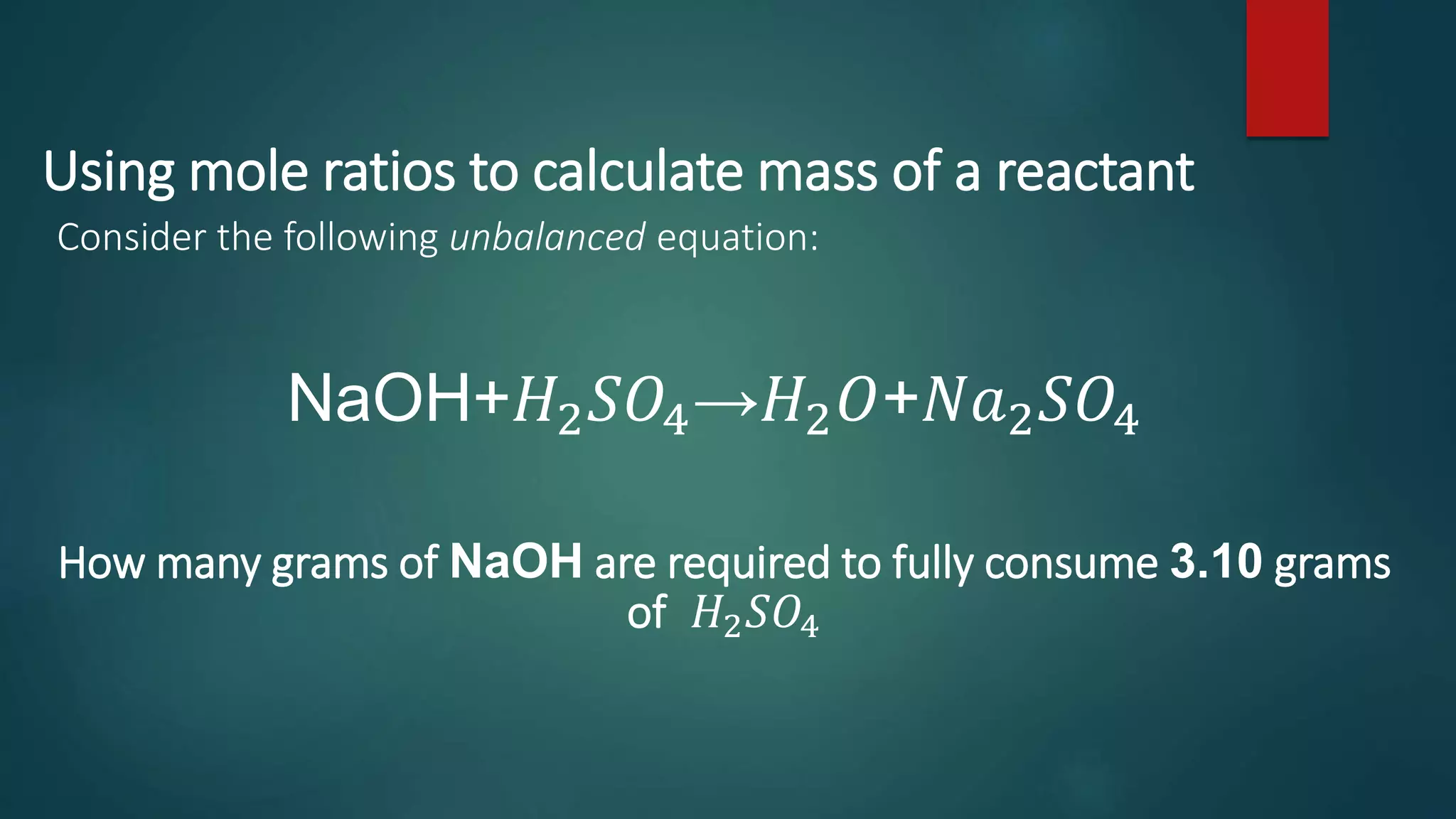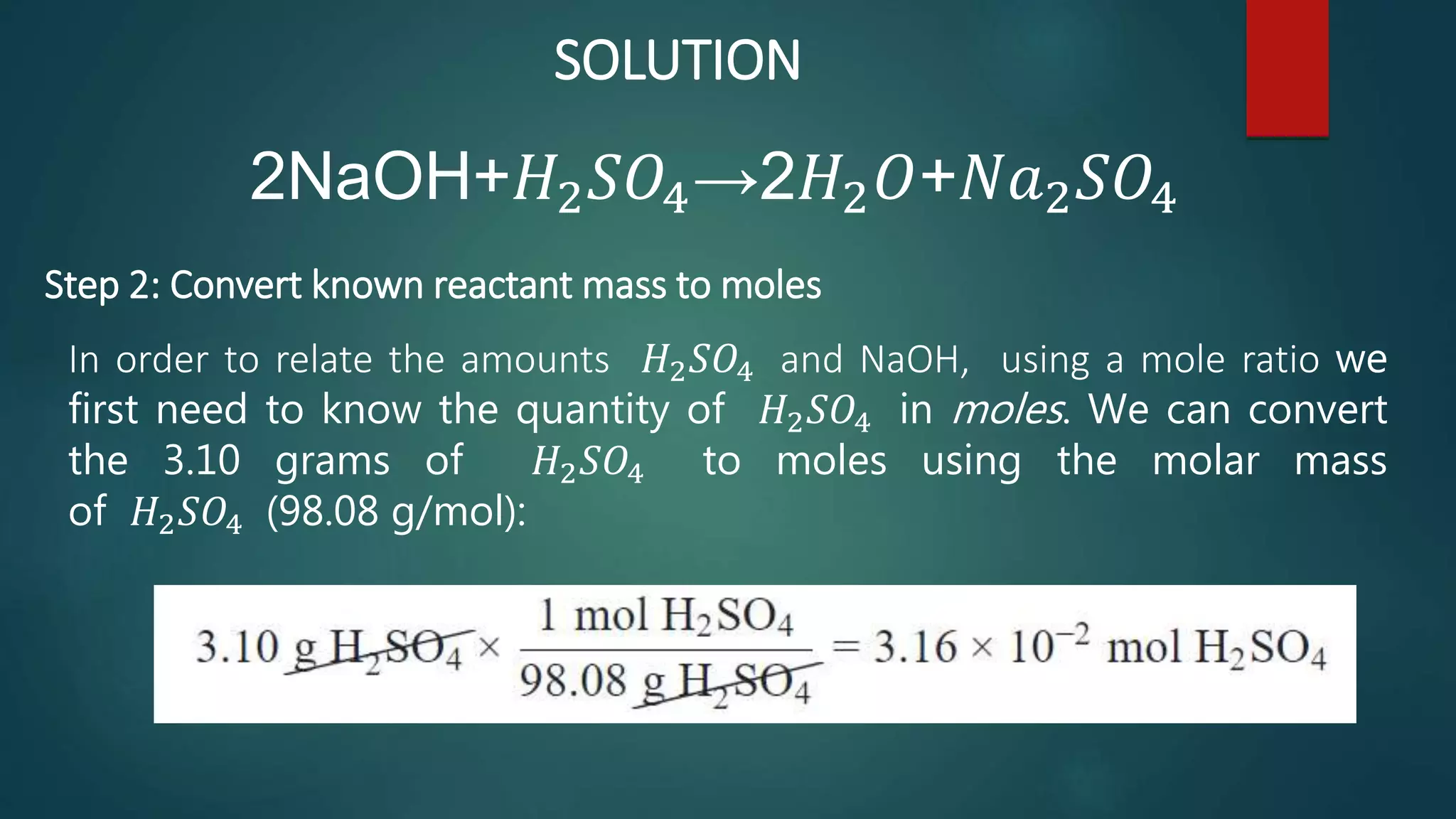The document discusses stoichiometry and using mole ratios to calculate amounts of substances in a chemical reaction. It provides an example of using the mole ratio from a balanced chemical equation between iron (III) oxide and aluminum to calculate how many moles of aluminum are needed to fully react with a given amount of iron (III) oxide. It also works through an example of using a mole ratio to calculate the grams of sodium hydroxide needed to fully react with 3.10 grams of sulfuric acid based on the balanced equation. Mole ratios allow conversion between amounts of any two substances involved in a chemical reaction.