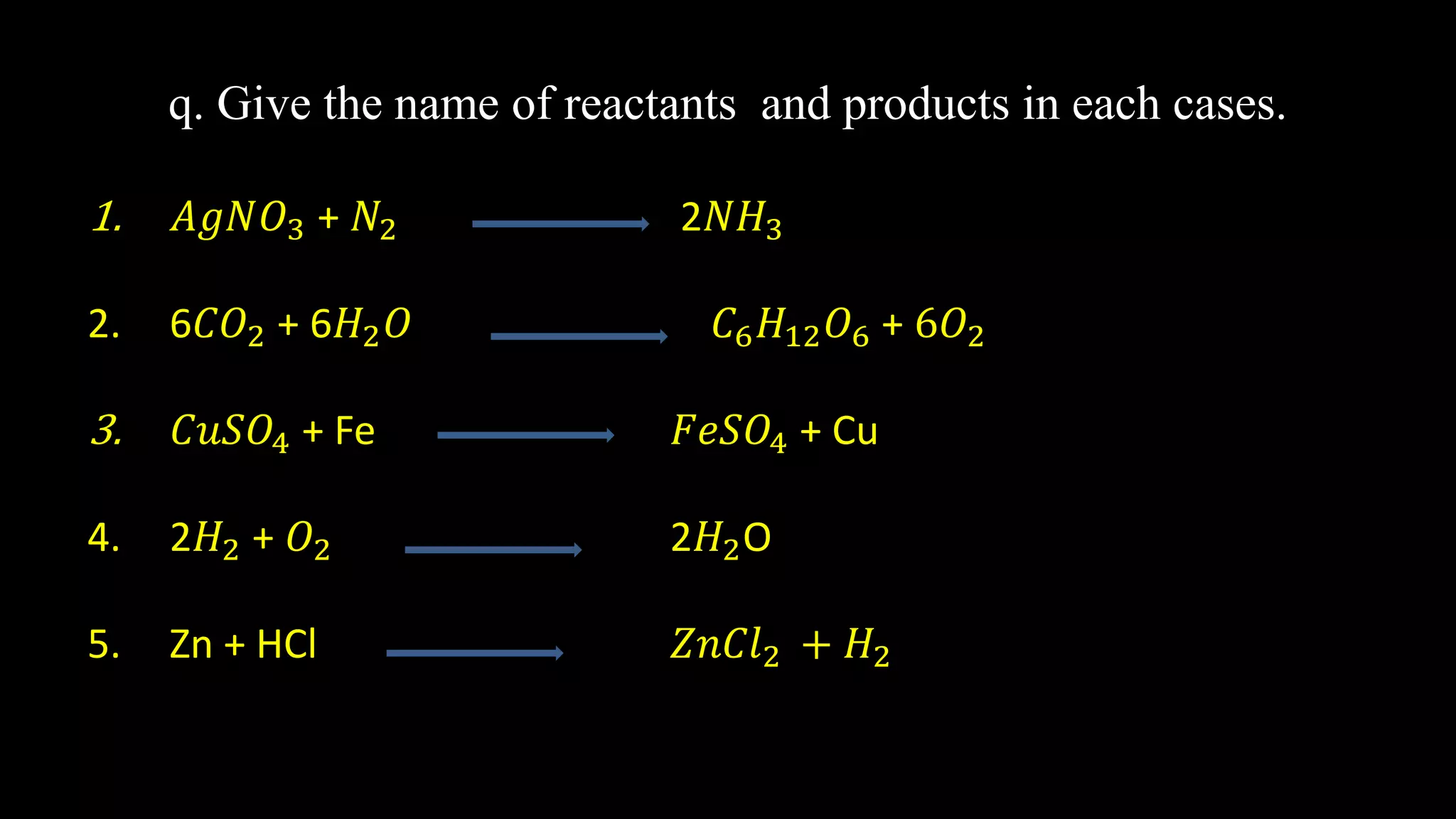
This document provides a summary of the syllabus for Chemistry (Science) for Class 10 as prescribed by the CBSE board. It covers the following key topics: 1) Chemical Reactions and Equations, 2) Acids, Bases and Salts, 3) Metals and Non-Metals, 4) Carbon and Its Compounds, and 5) Periodic Classification of Elements. The first chapter focuses on chemical reactions, equations, types of chemical reactions including combination, decomposition, displacement and redox reactions. It also discusses examples of chemical reactions in everyday life such as photosynthesis, combustion and corrosion.