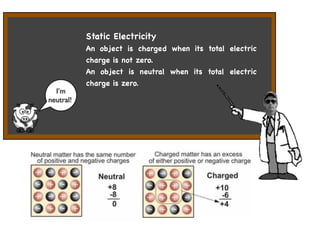
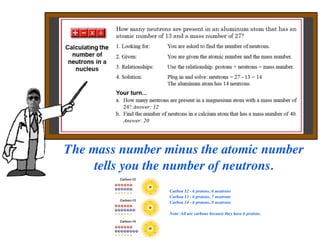
The document discusses the fundamental particles and forces that make up atoms. It describes how atoms were discovered to be made up of even smaller particles, including electrons discovered by J.J. Thomson and the nuclear model developed by Rutherford based on experiments by Rutherford, Geiger, and Marsden. The document also discusses the three main subatomic particles (protons, neutrons, and electrons), isotopes, radioactive decay, and the four main forces (electromagnetic, strong nuclear, weak, and gravity) that act inside atoms.