This document discusses atomic structure and properties. It defines:
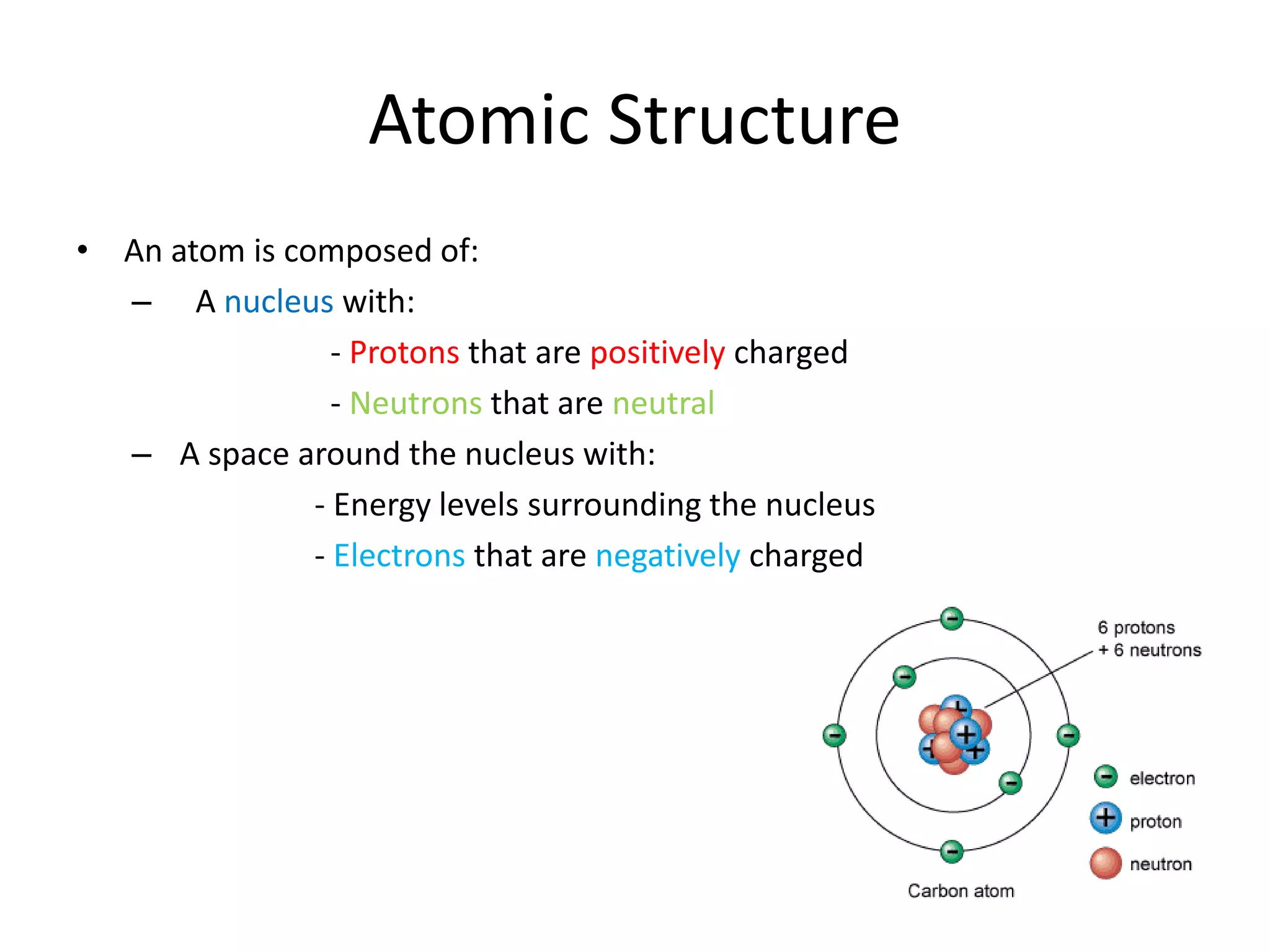
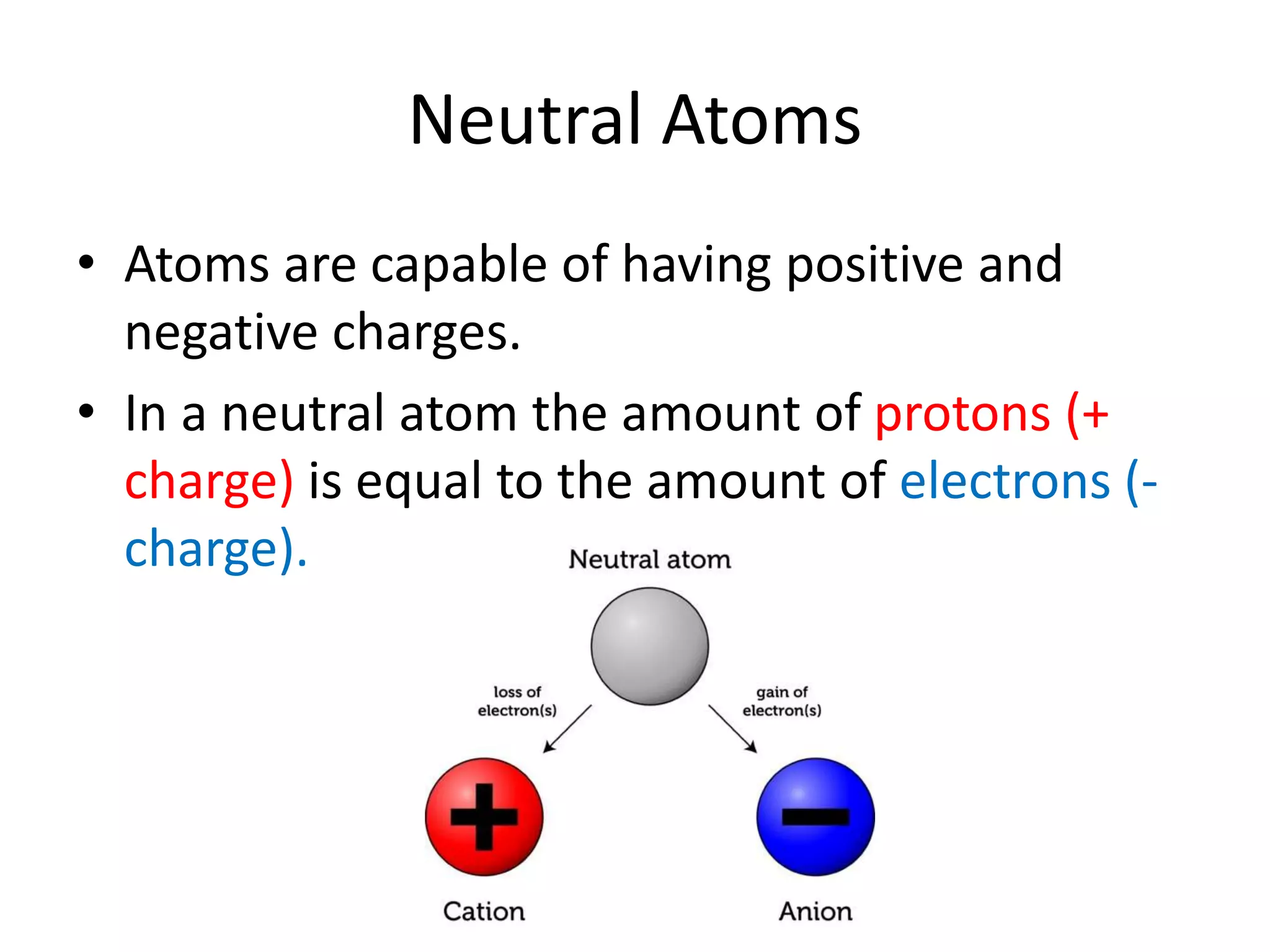
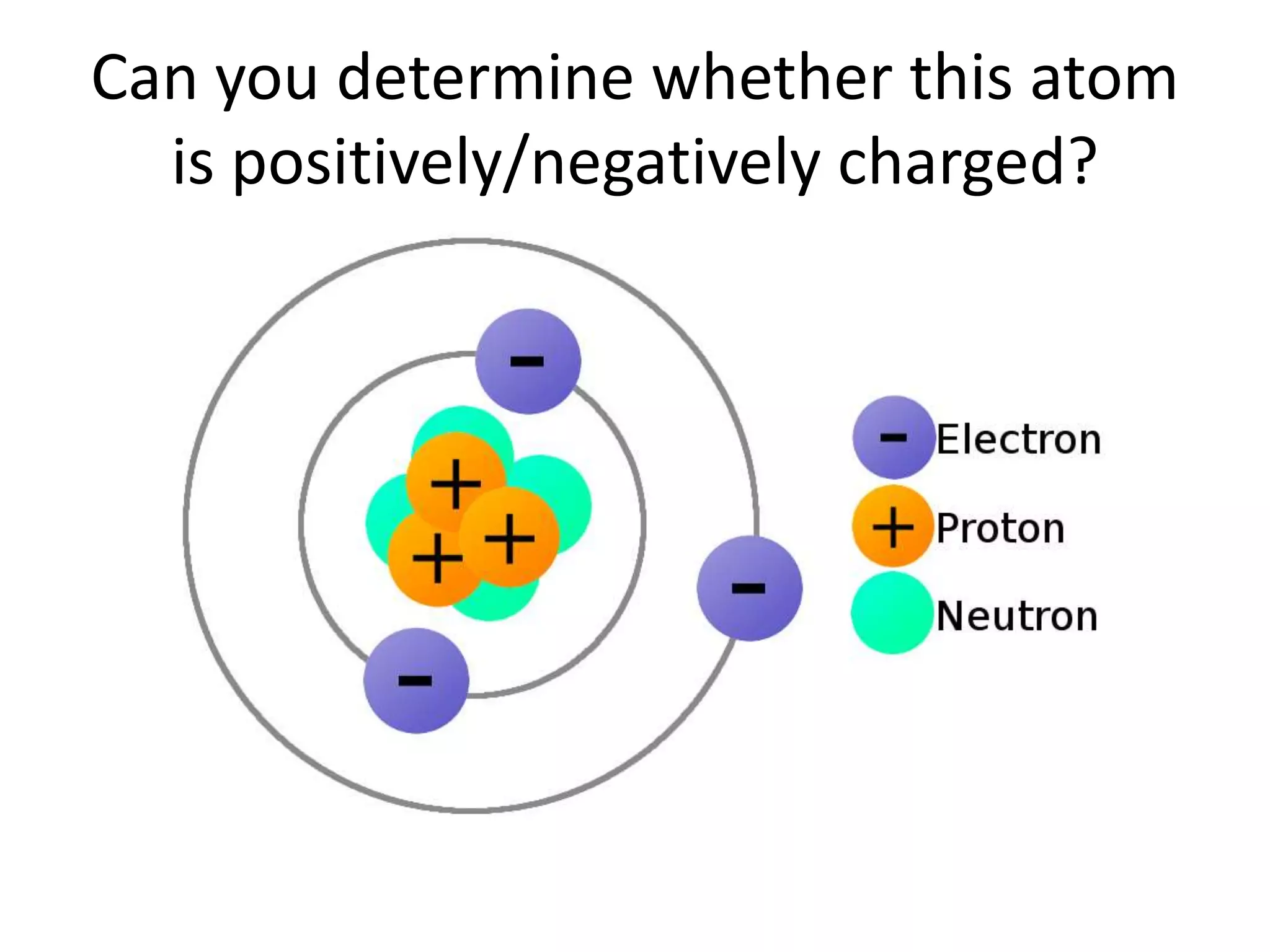
- Atoms consist of a nucleus with protons and neutrons, and electrons orbiting the nucleus.
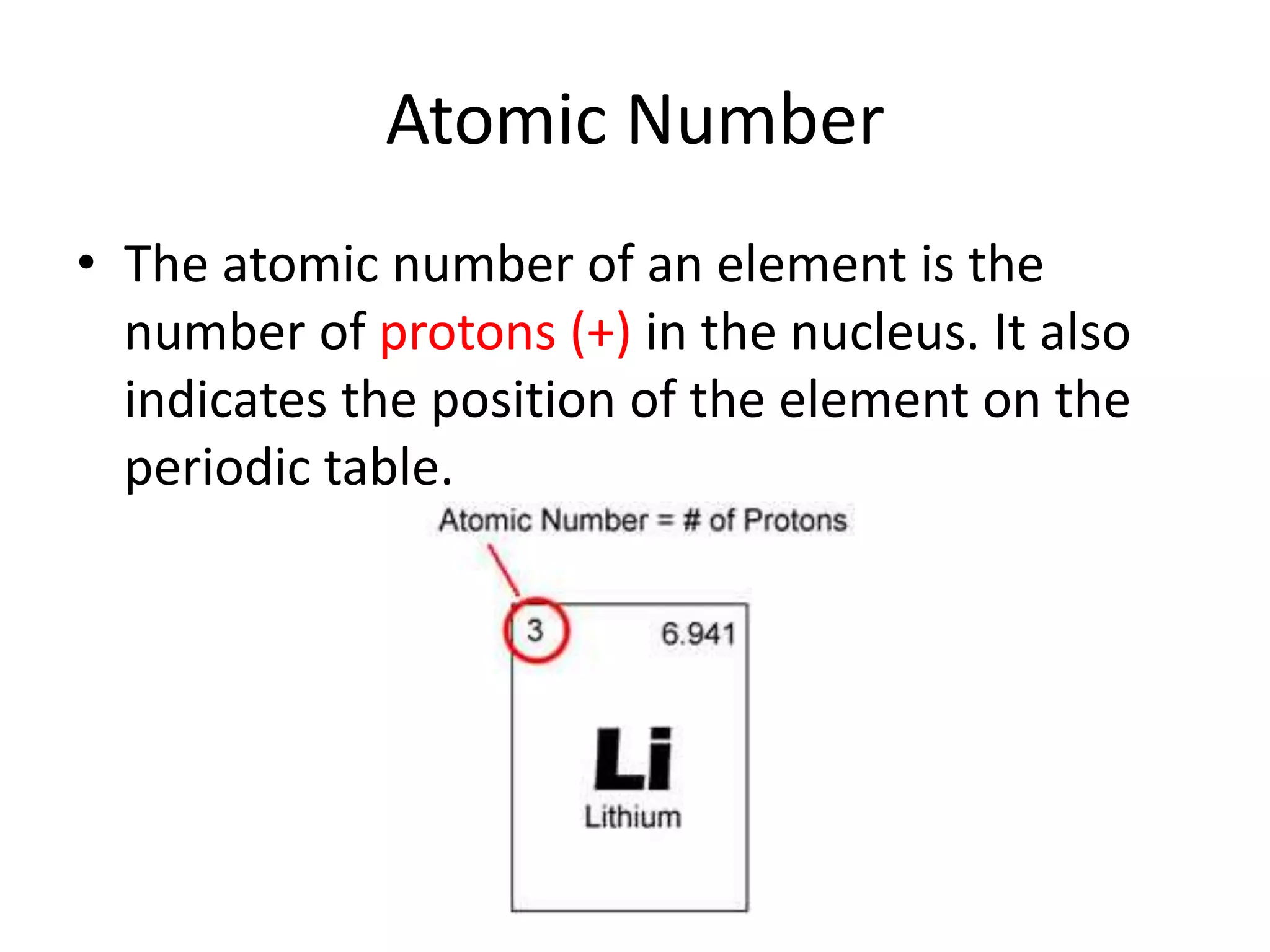
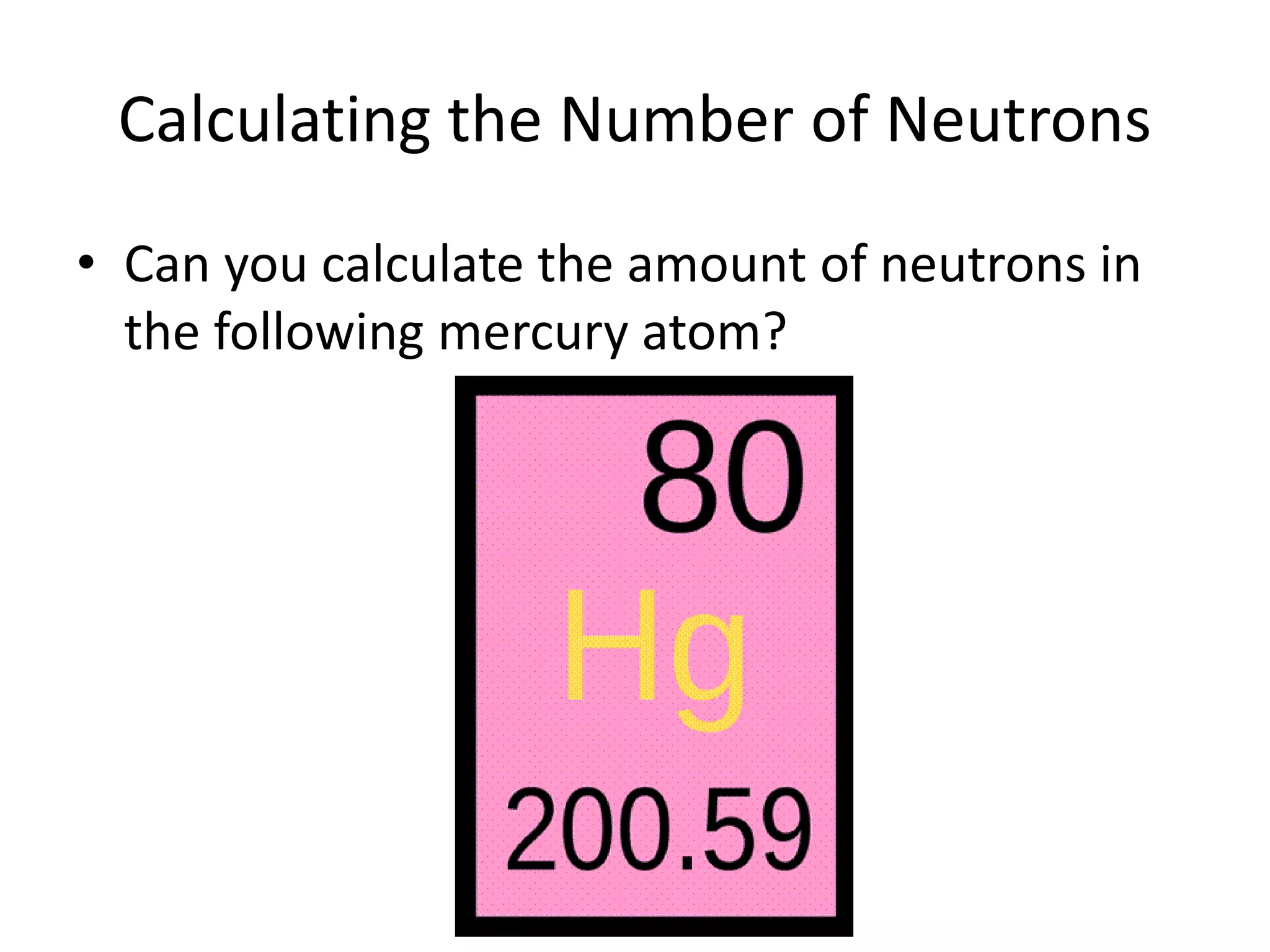
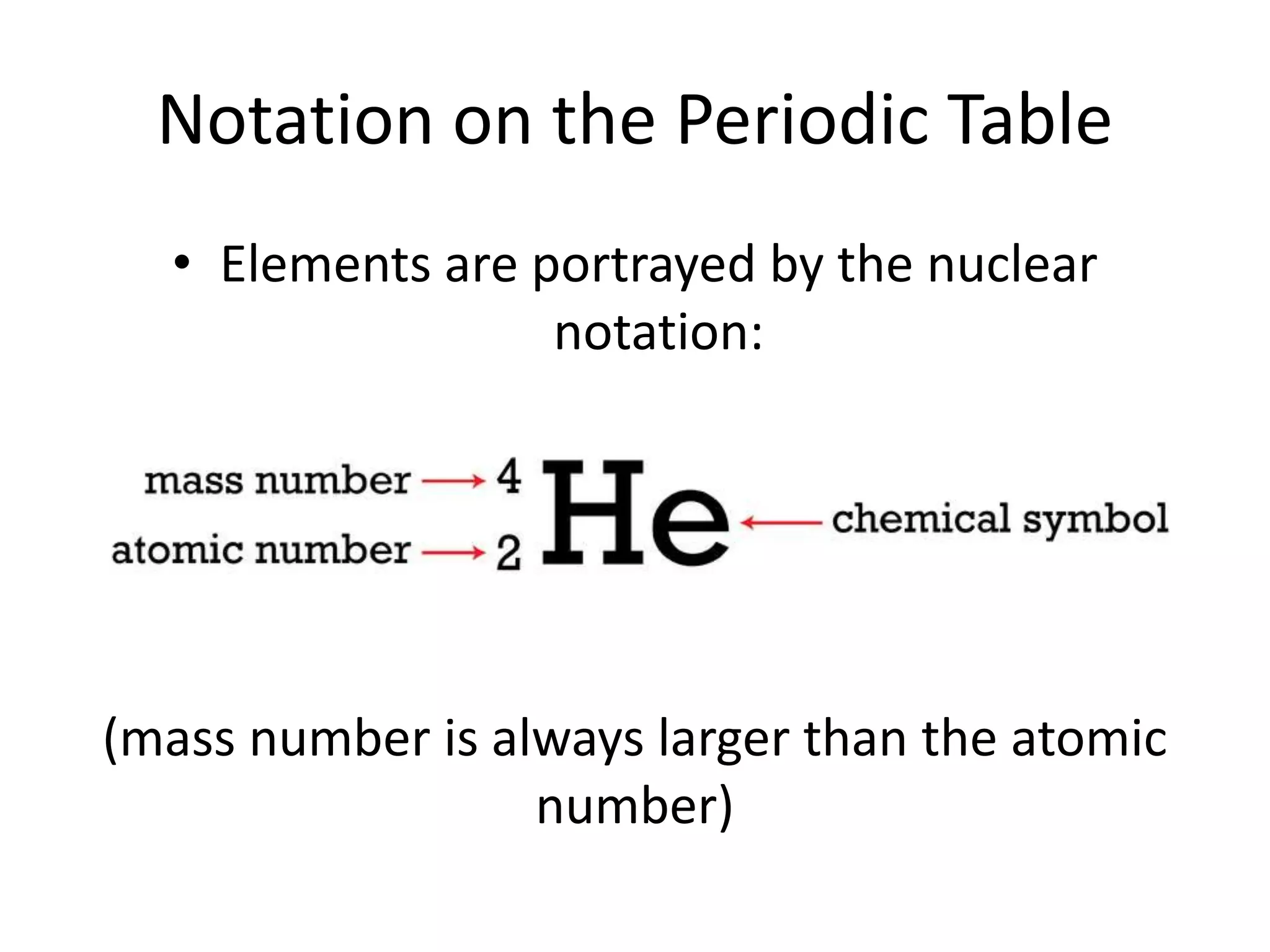
- The atomic number is the number of protons, which identifies an element and its position on the periodic table.
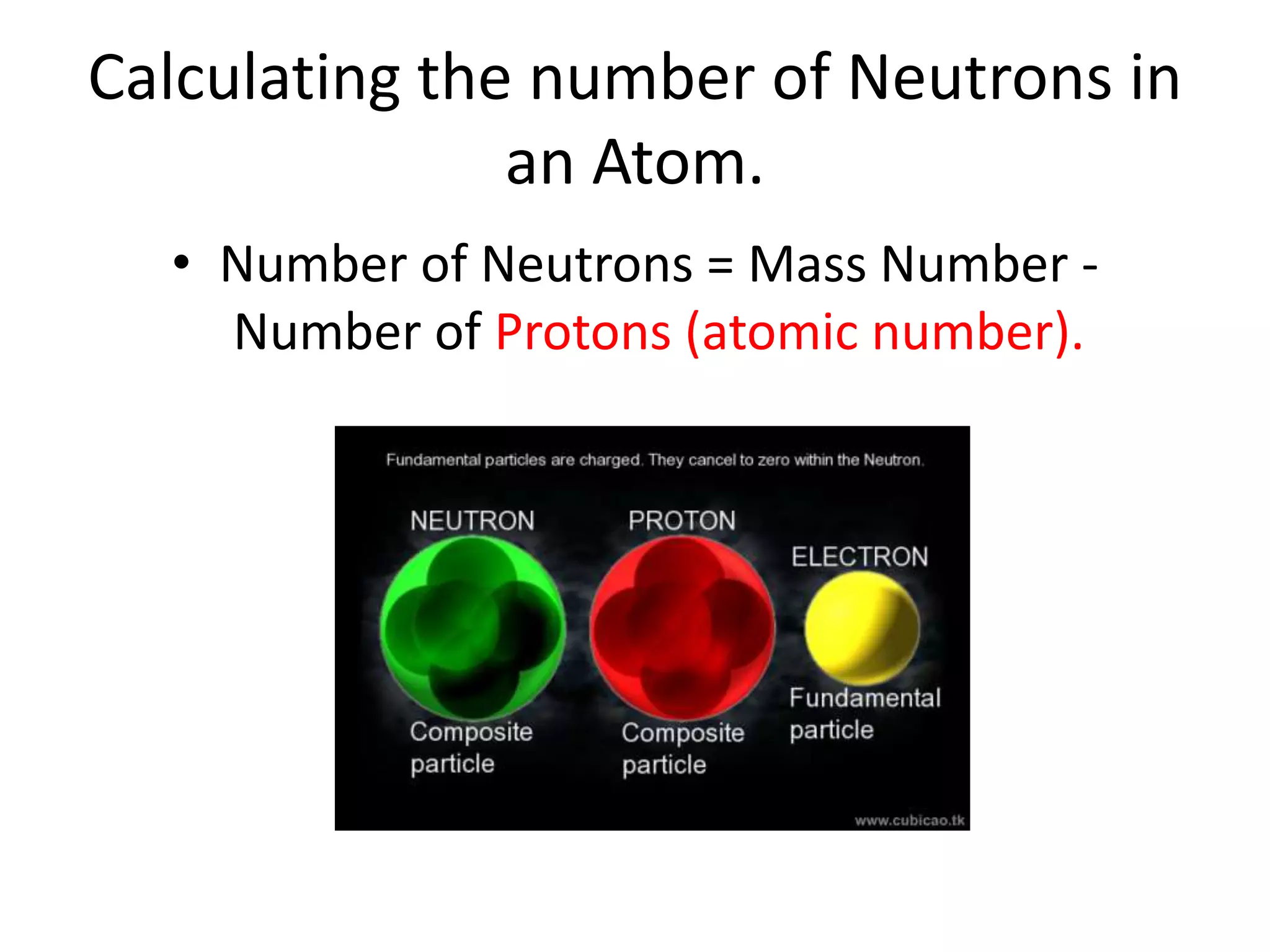
- Neutrons have no charge and contribute to an atom's mass.
- The mass number is the total number of protons and neutrons, and provides an indication of an atom's mass.