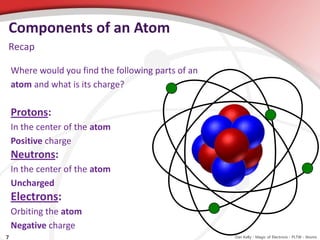
Atoms are the smallest particles of matter that make up everything, with elements consisting of only one type of atom and compounds comprising different types of atoms. Atoms consist of a nucleus containing protons and neutrons, with electrons orbiting around it, and the atomic number denotes the number of protons in the nucleus. Understanding electrons is crucial for grasping the principles of electricity.