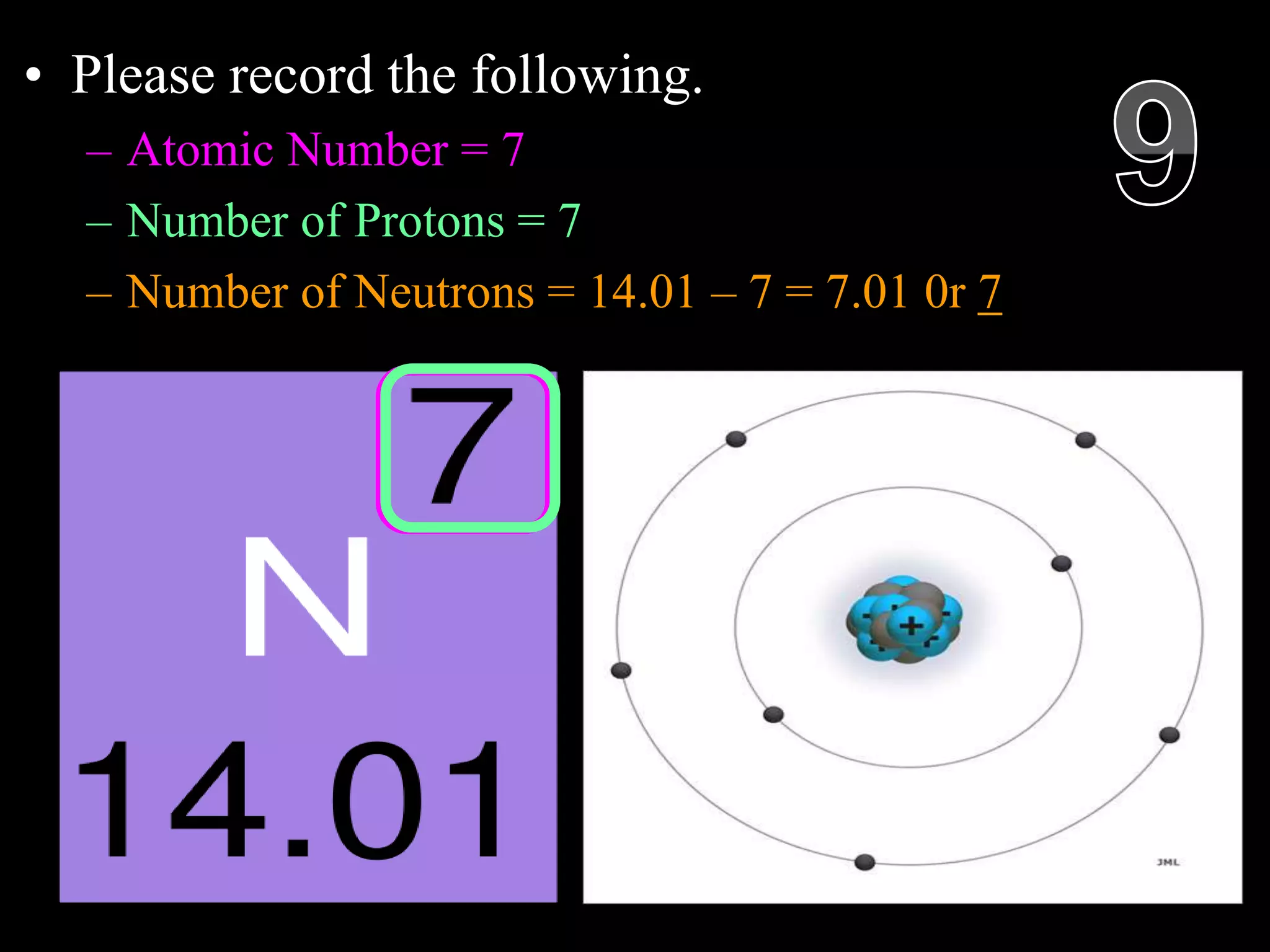
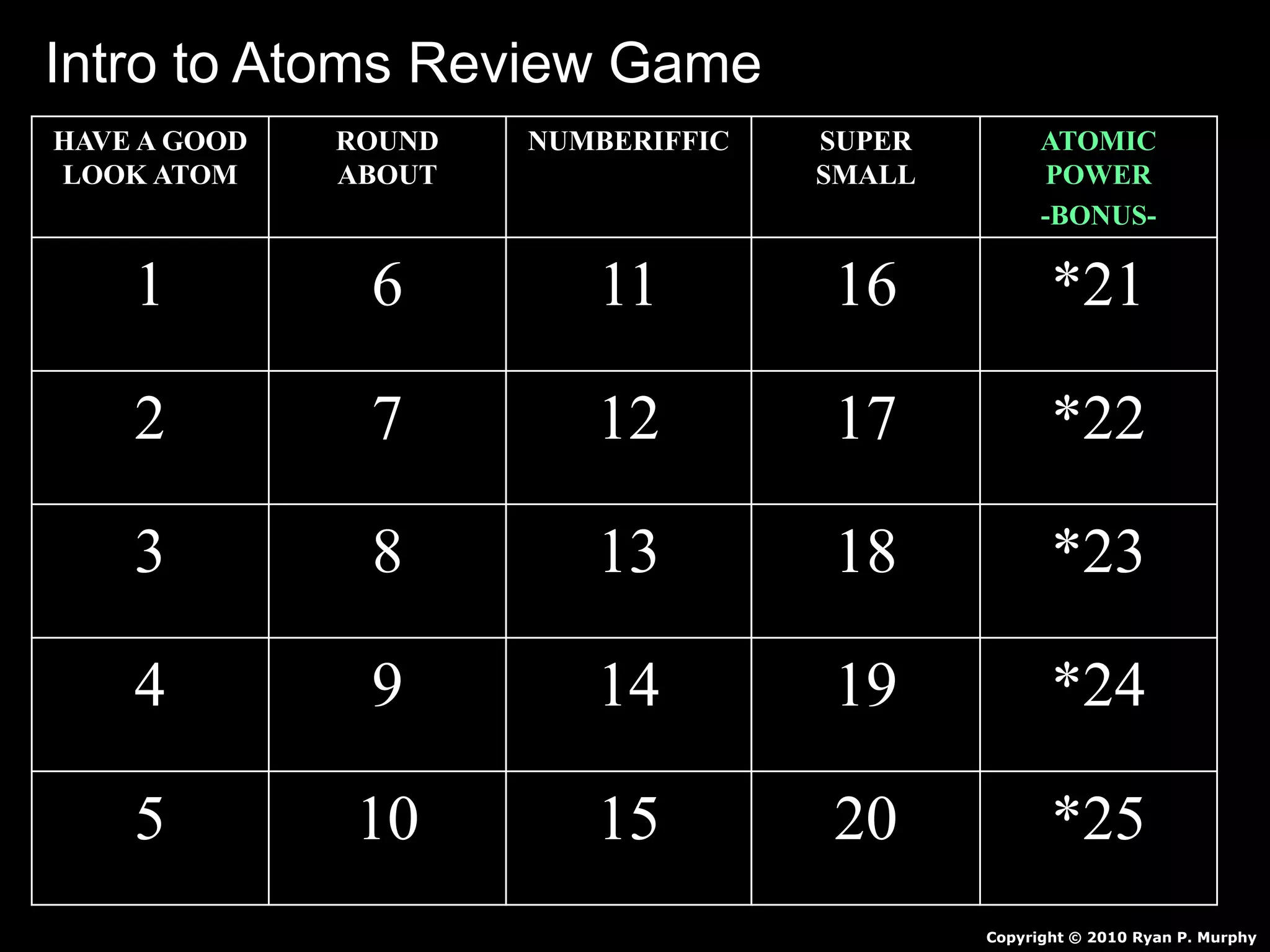
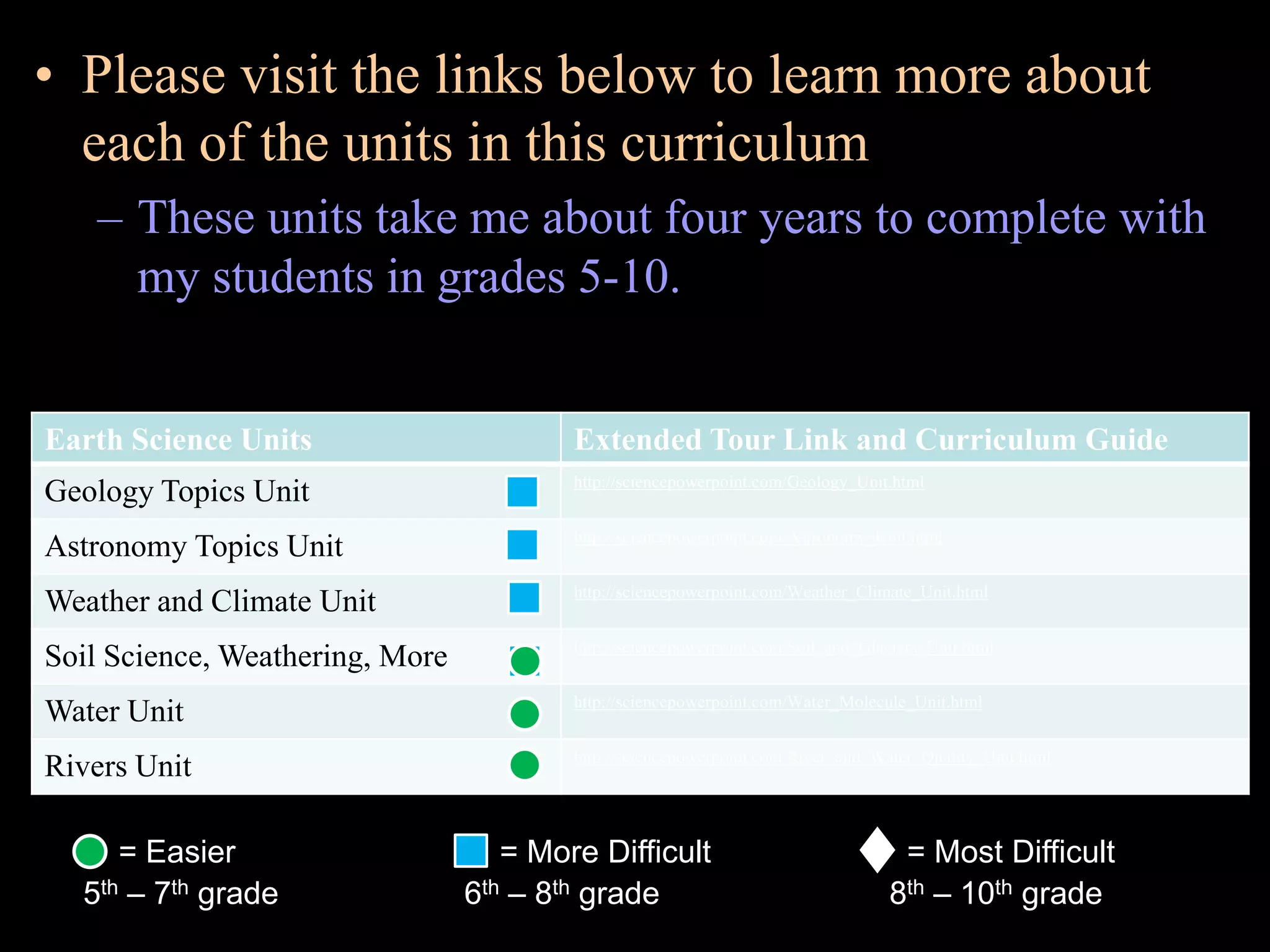
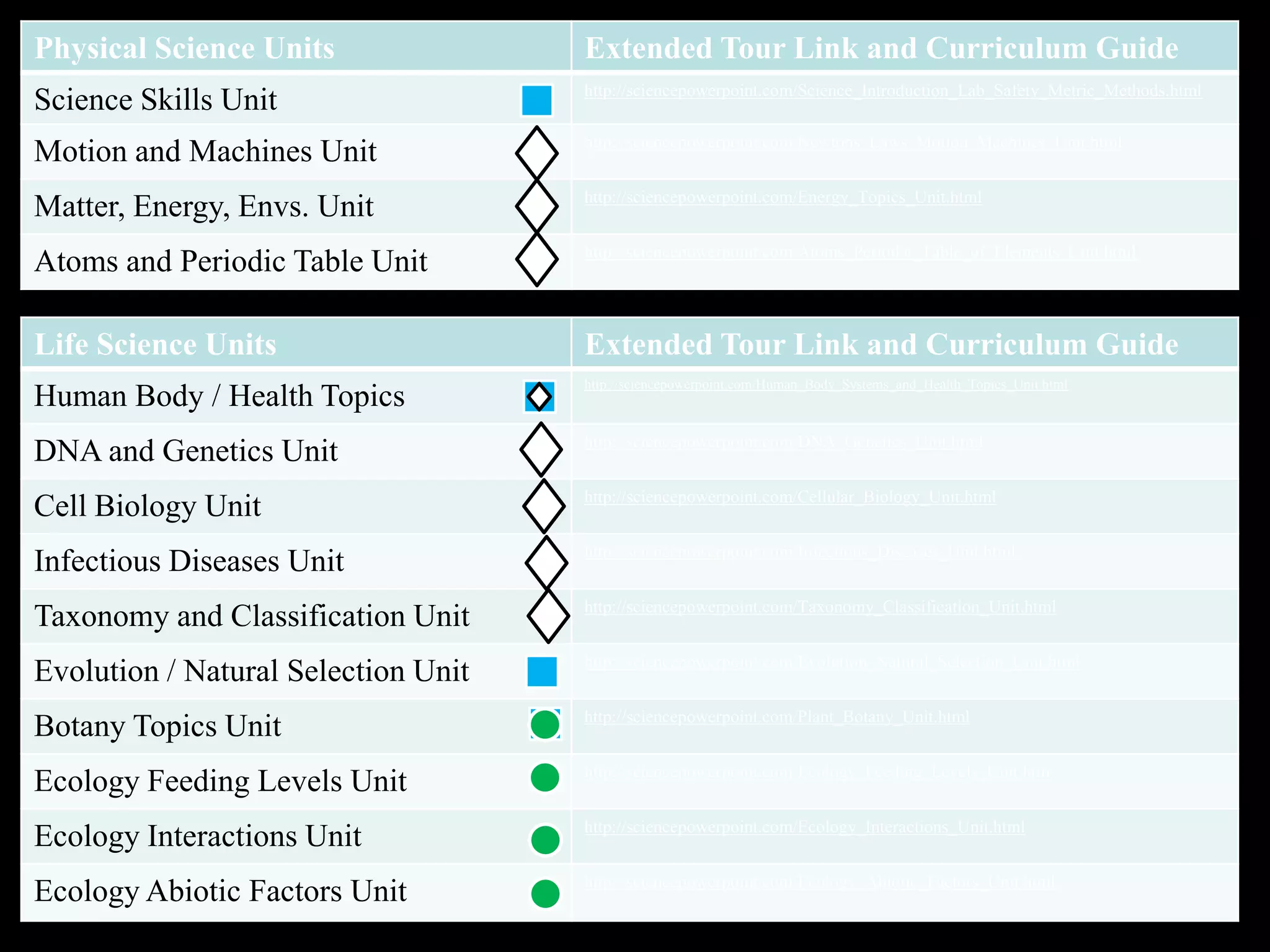
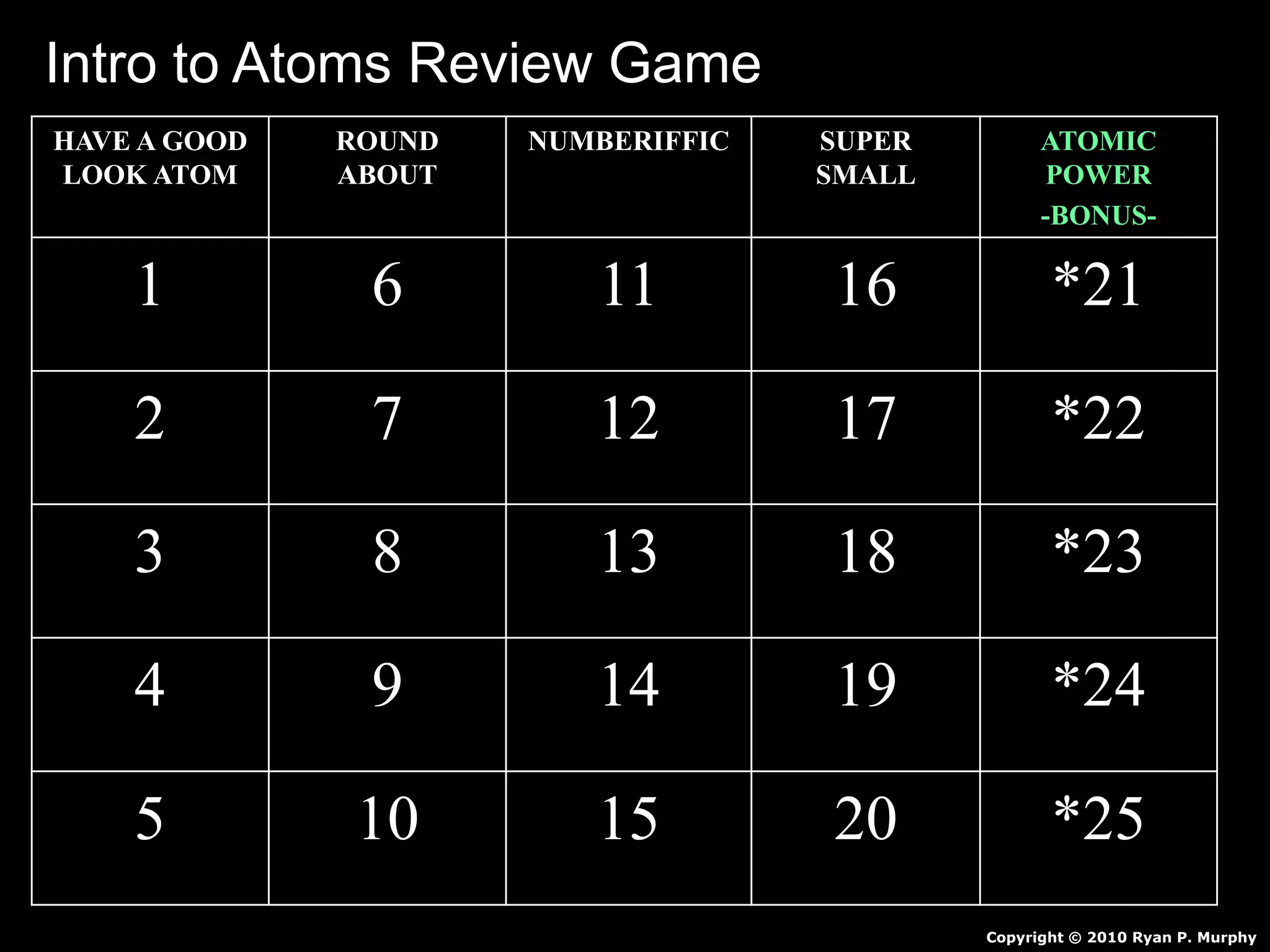
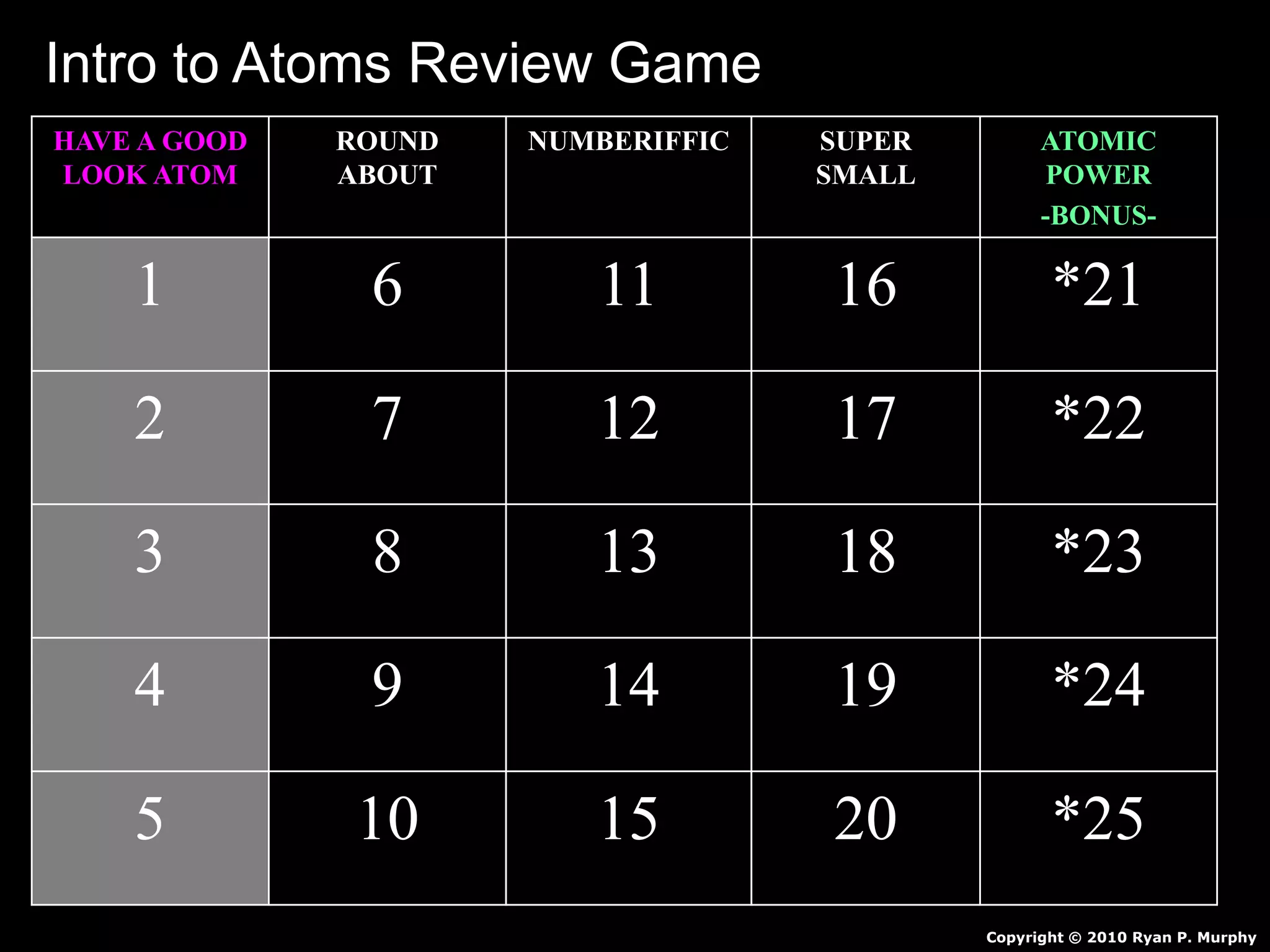
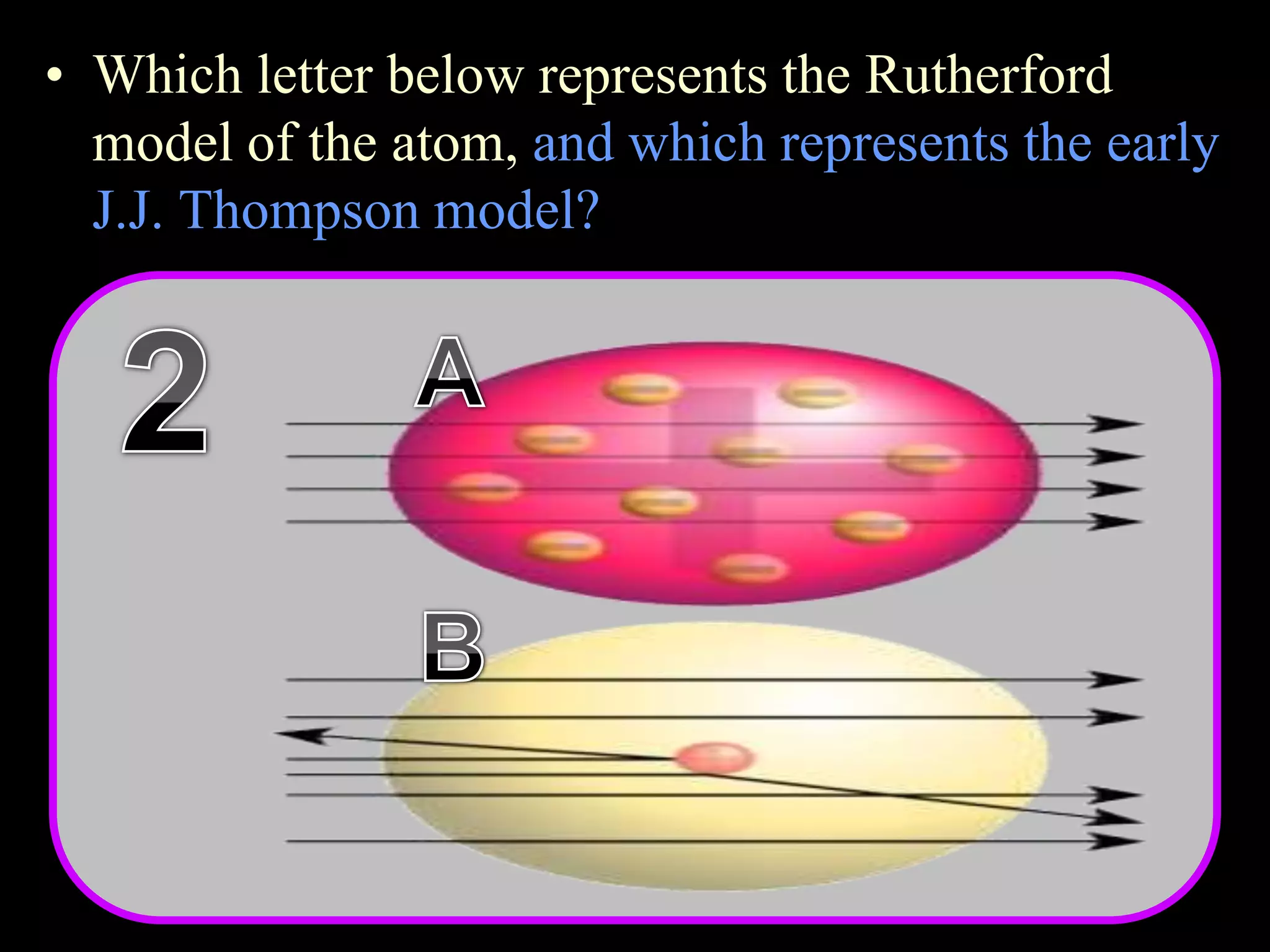
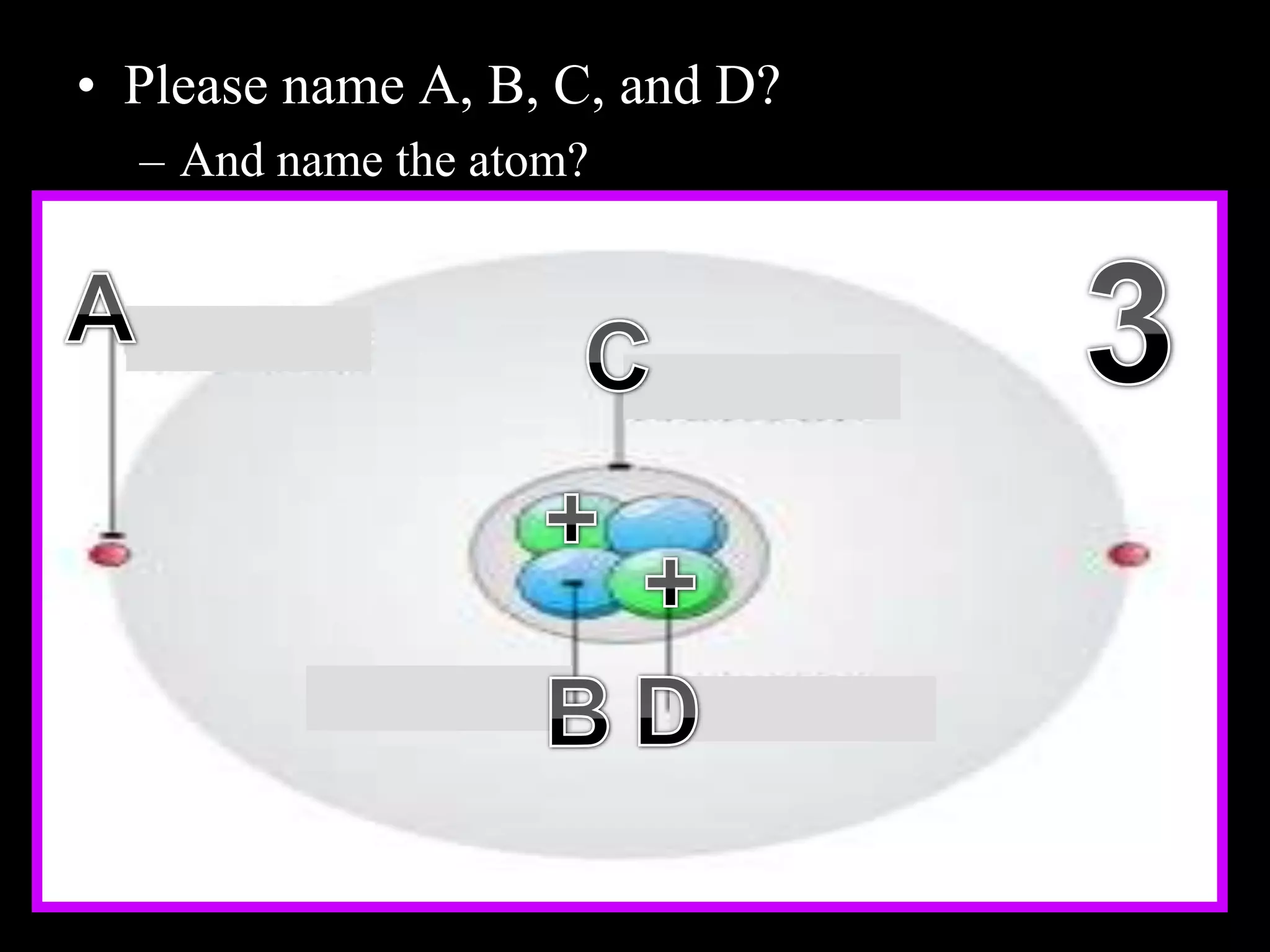
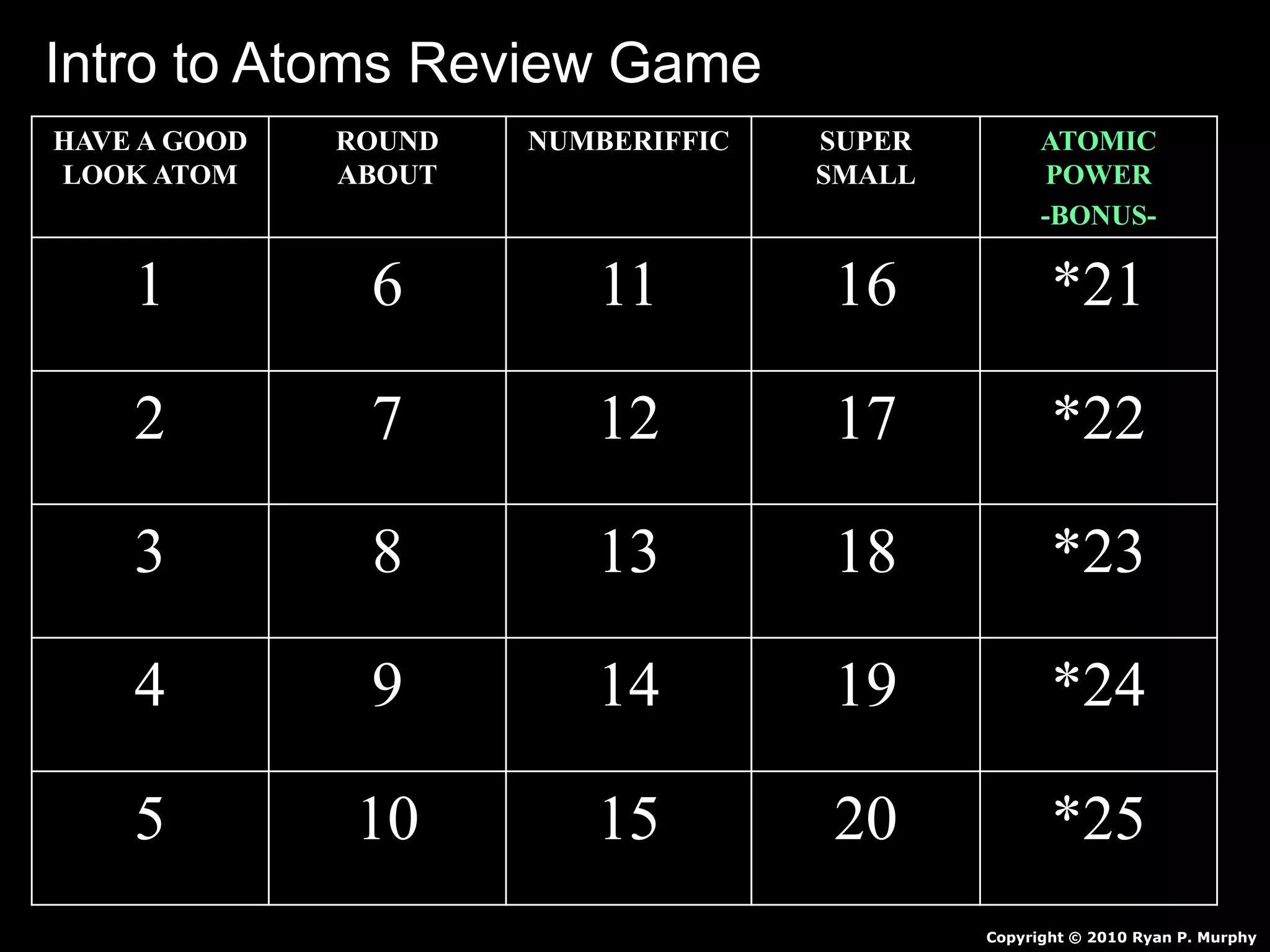
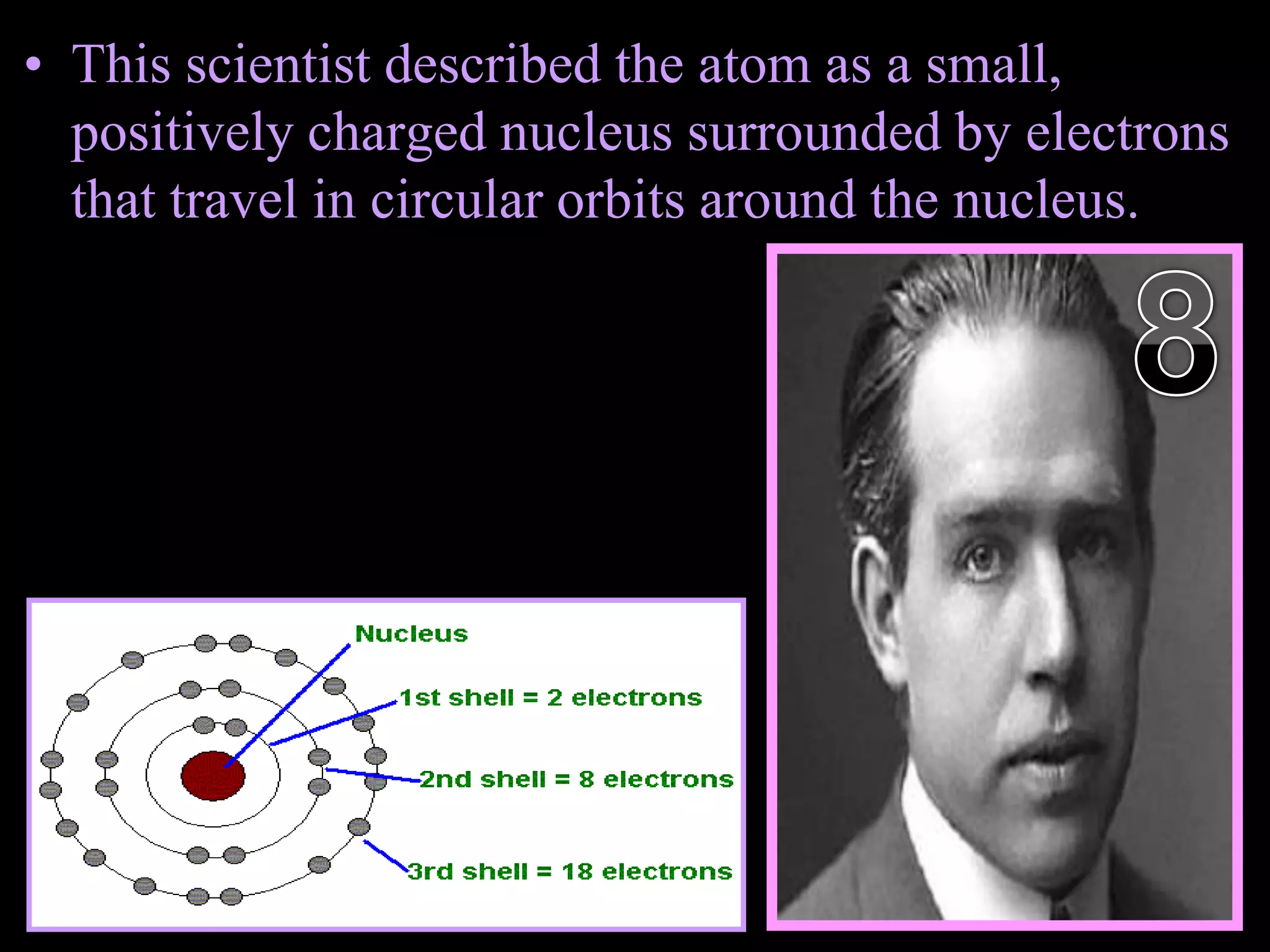
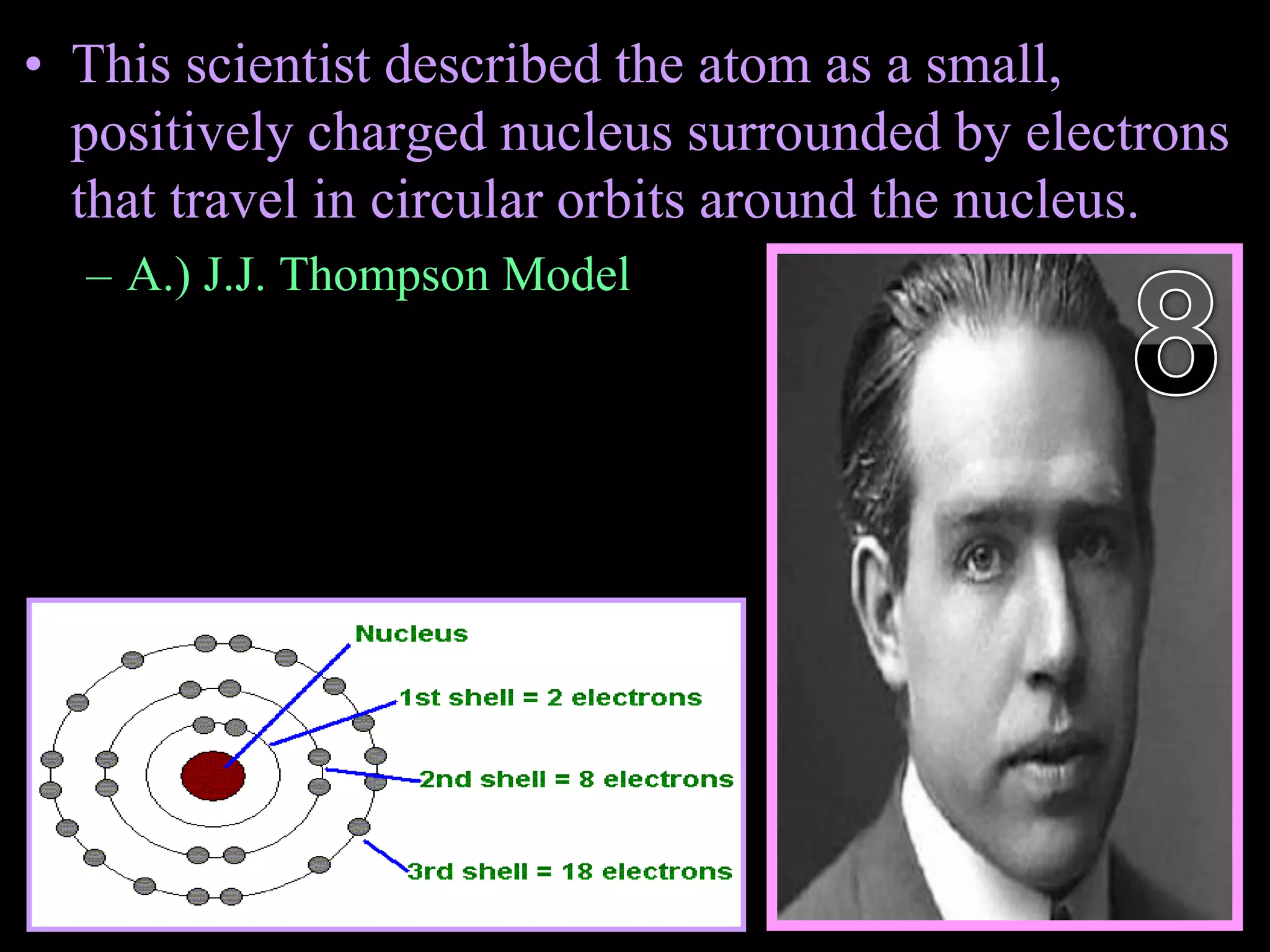
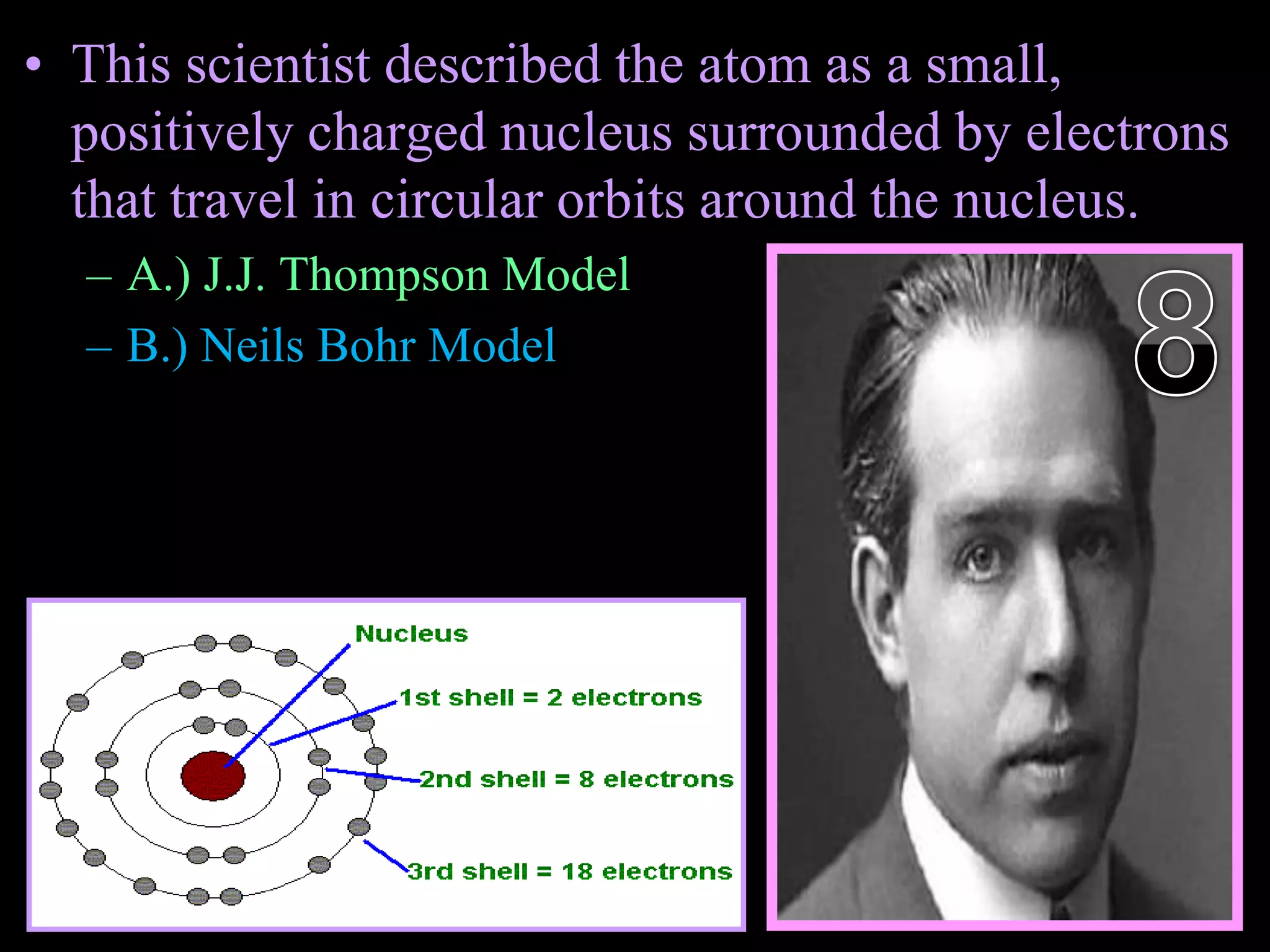
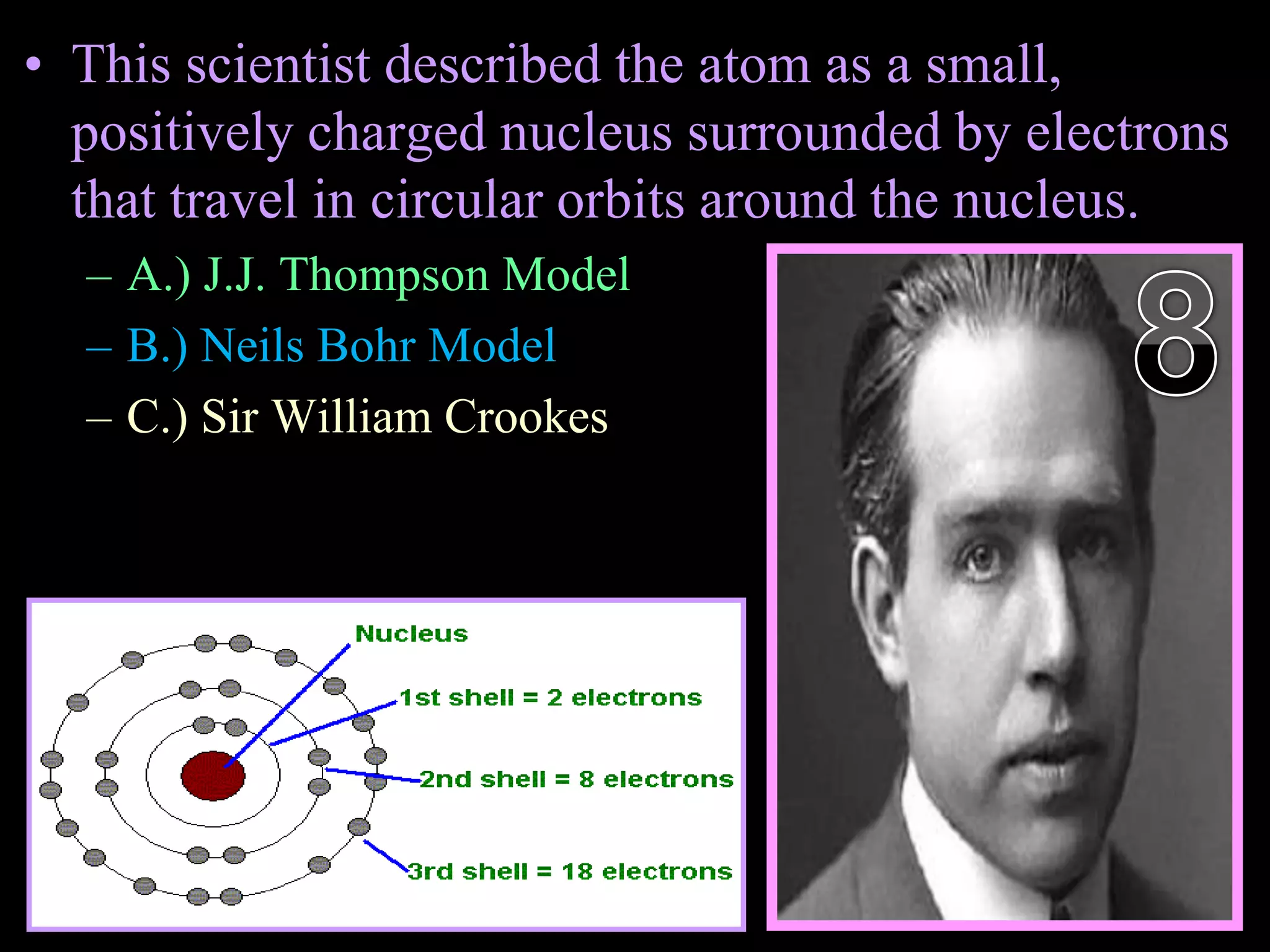
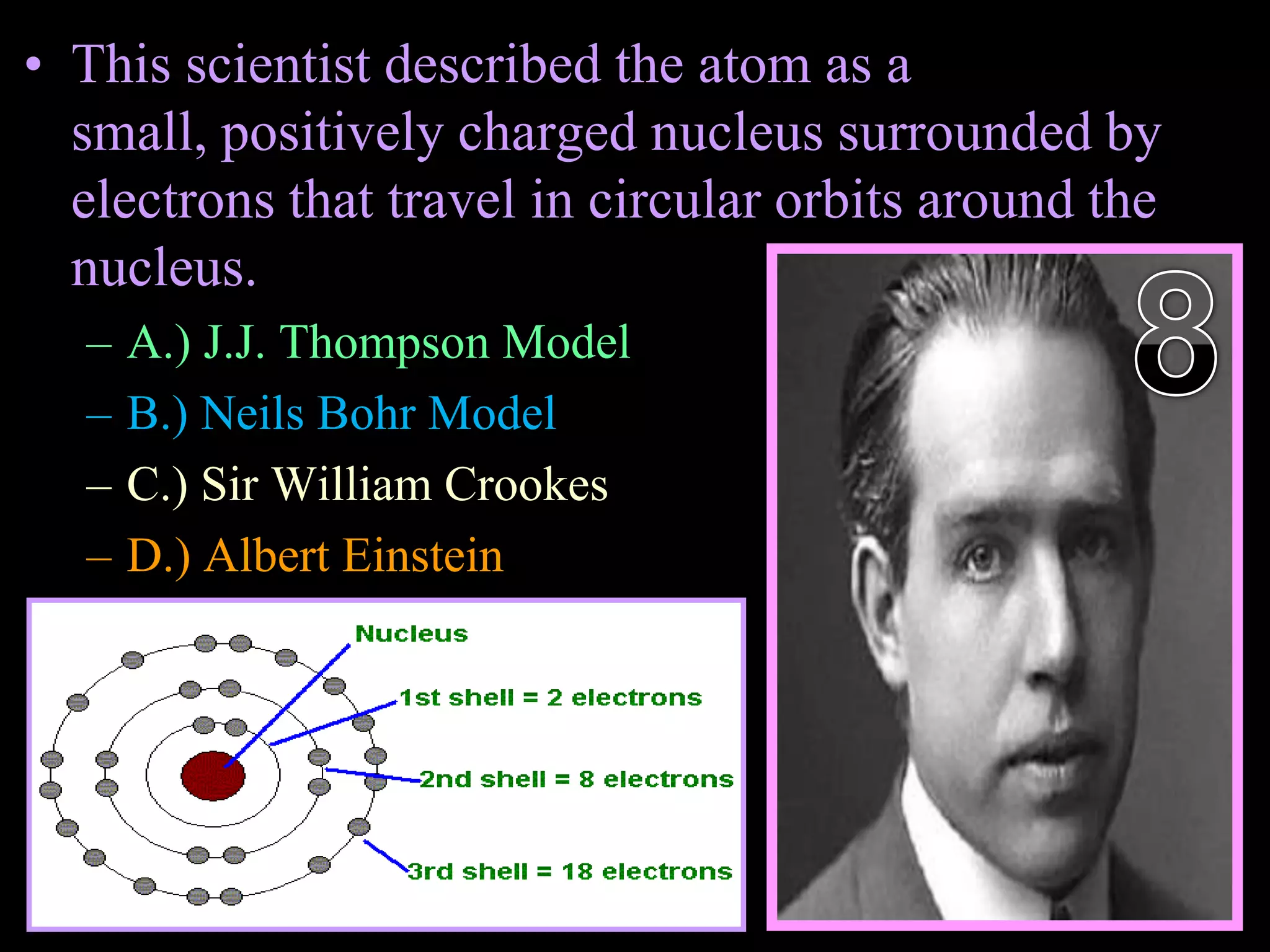
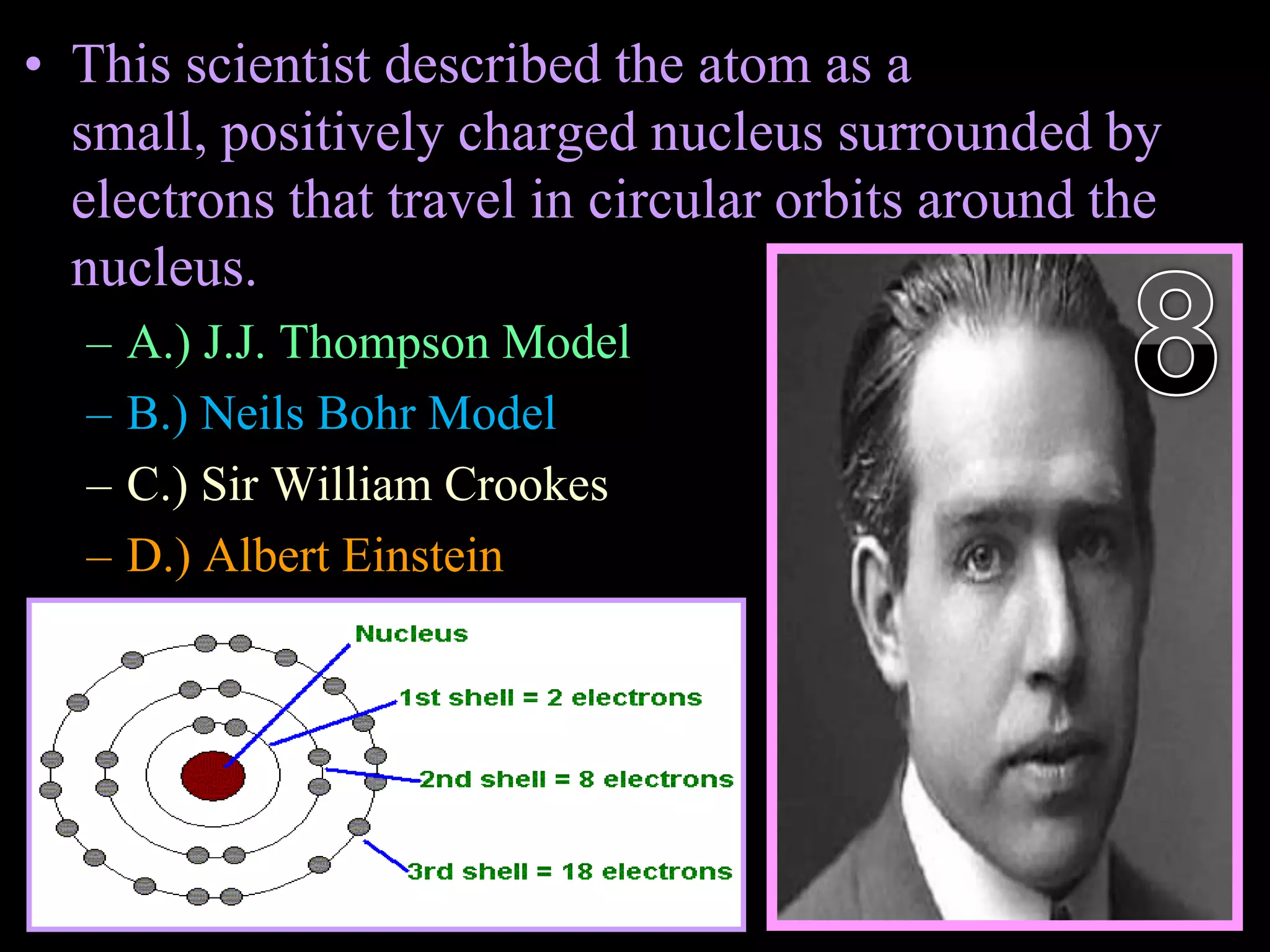
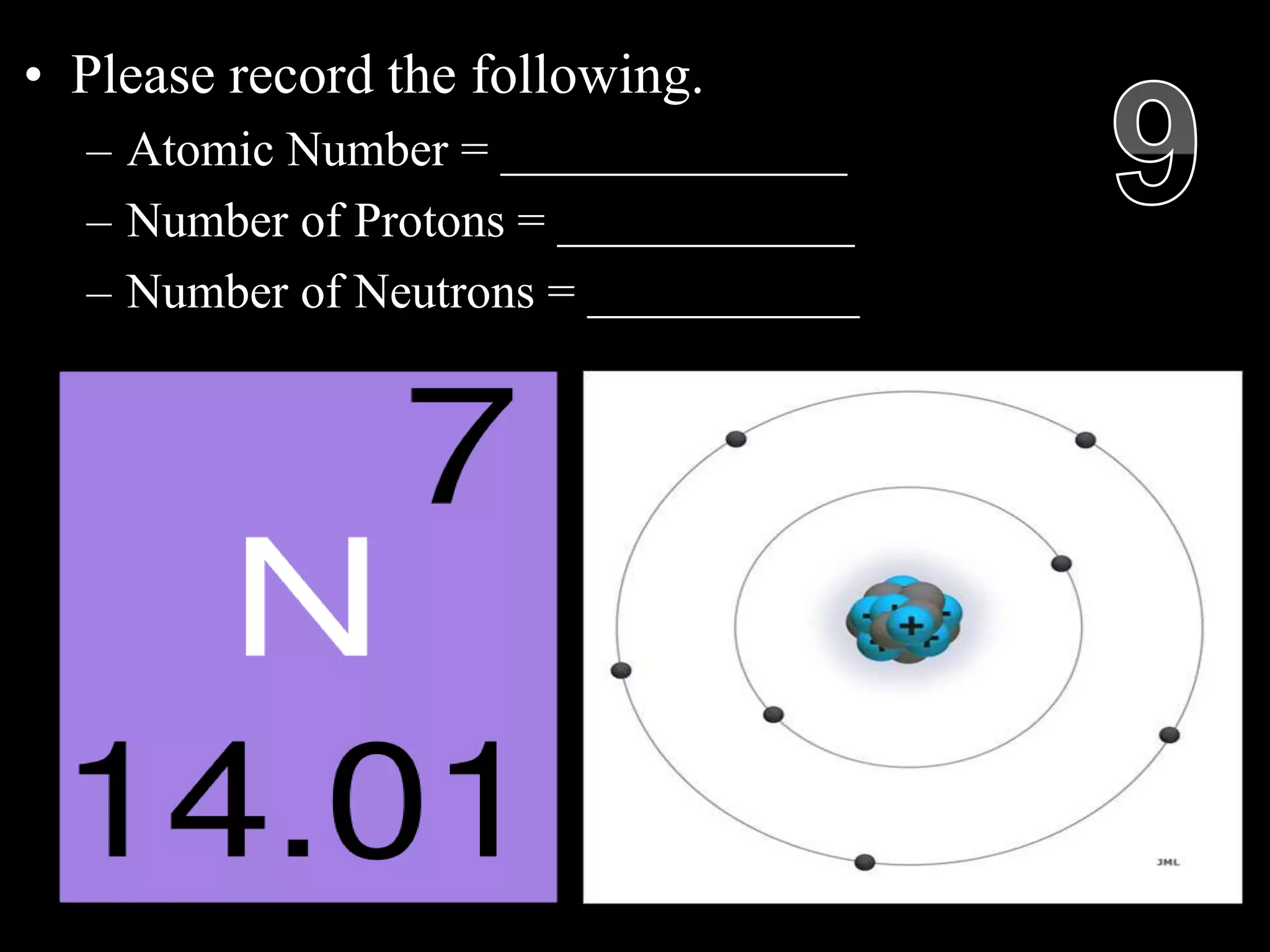
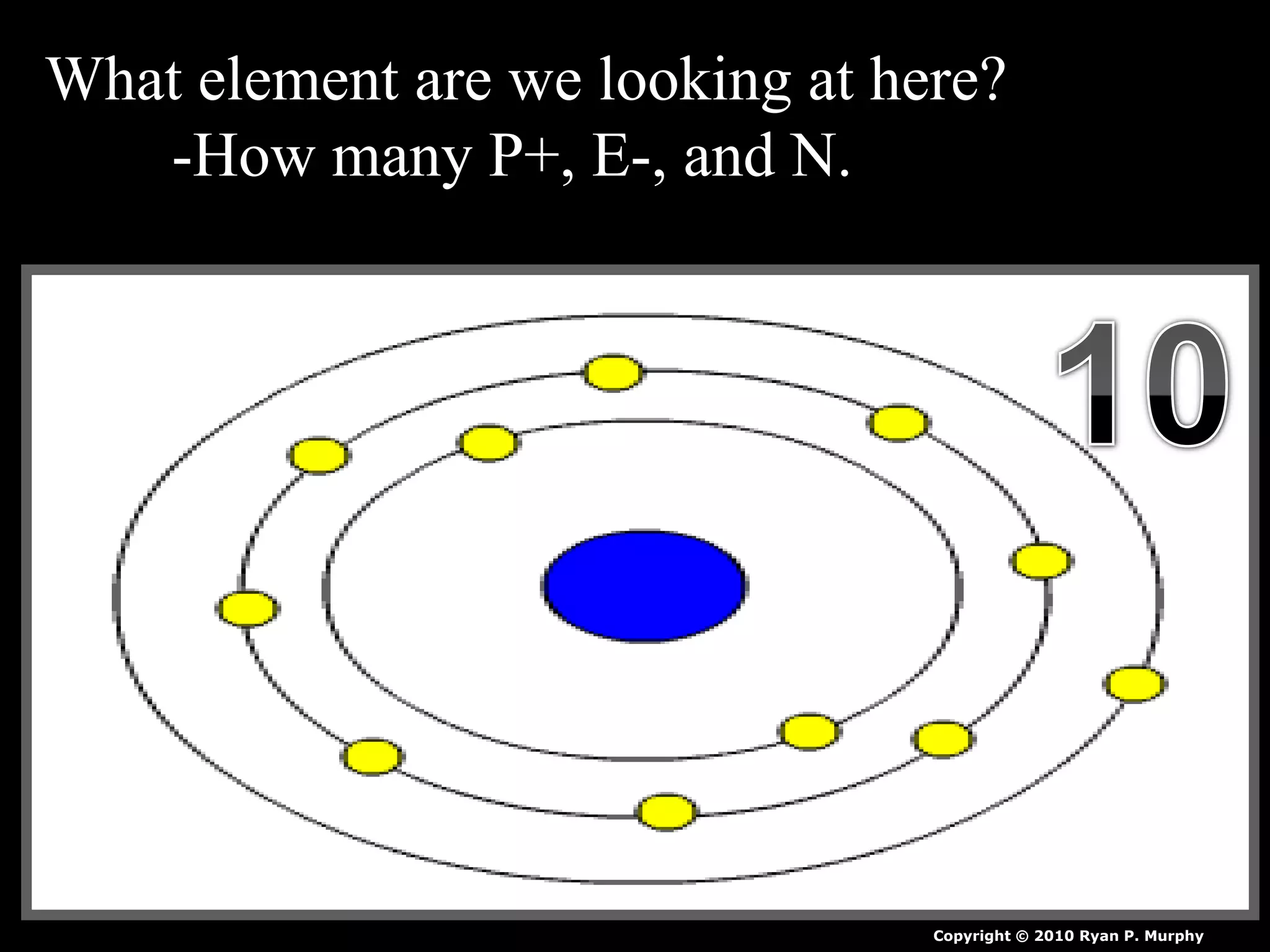
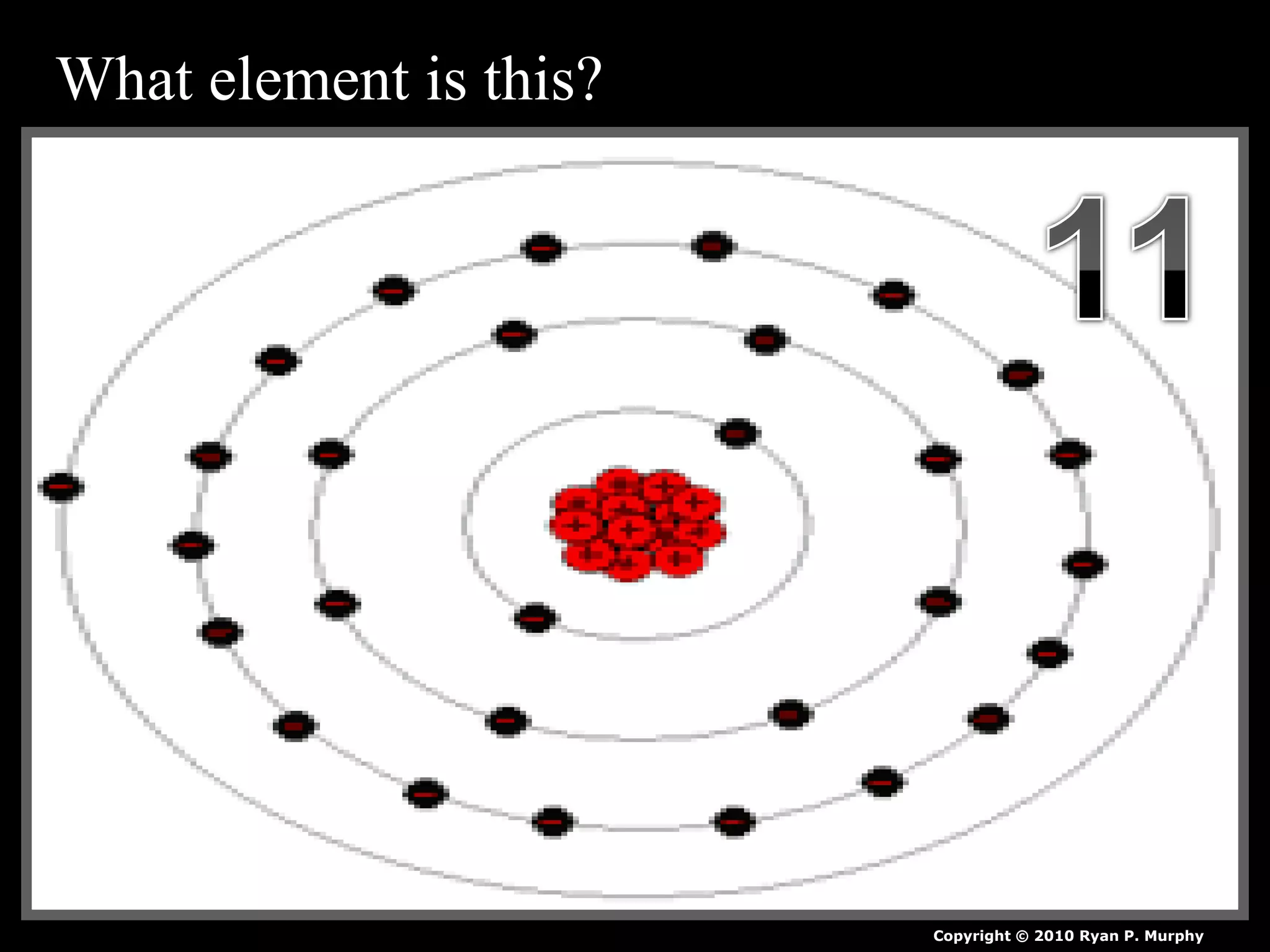
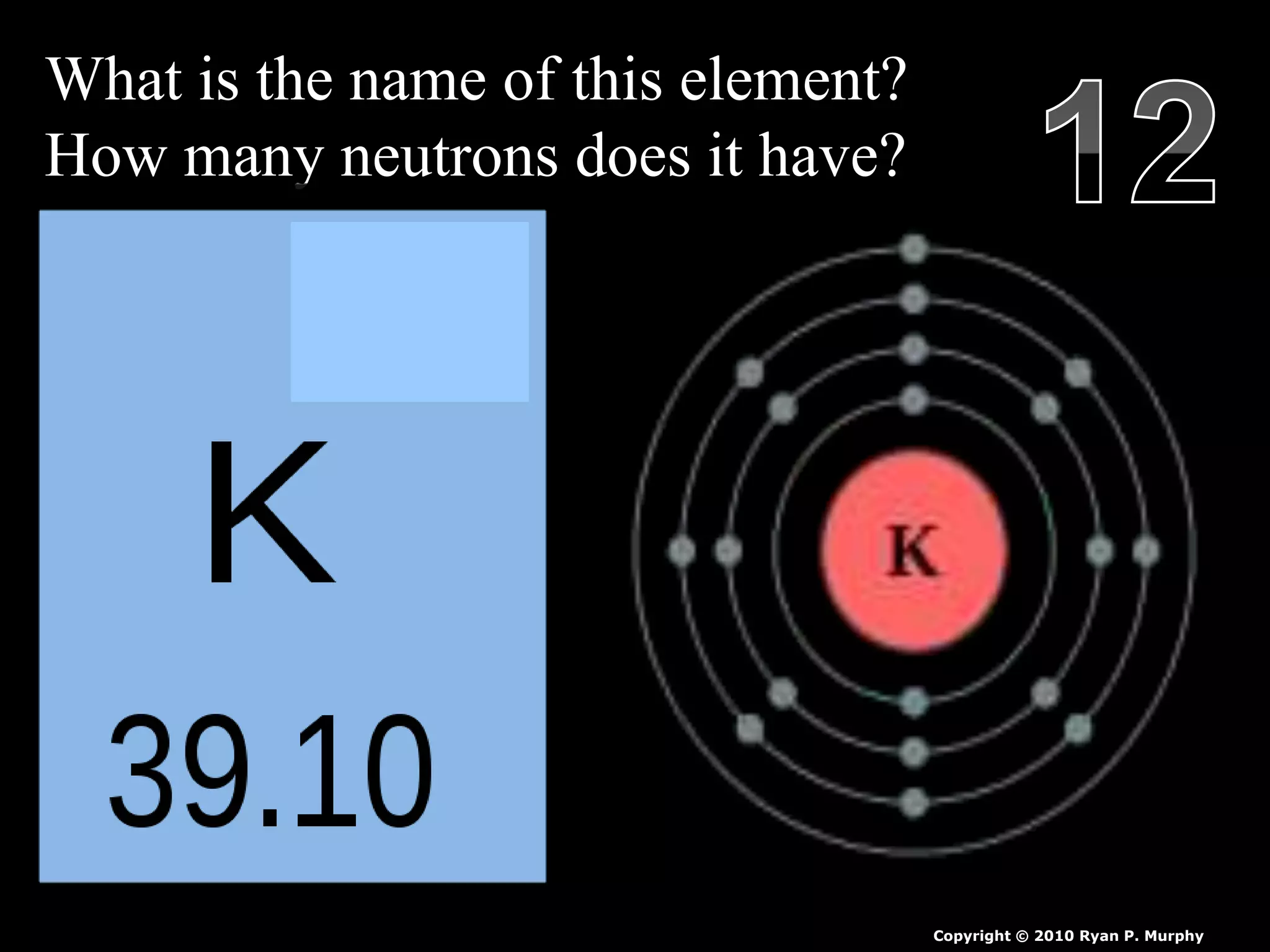
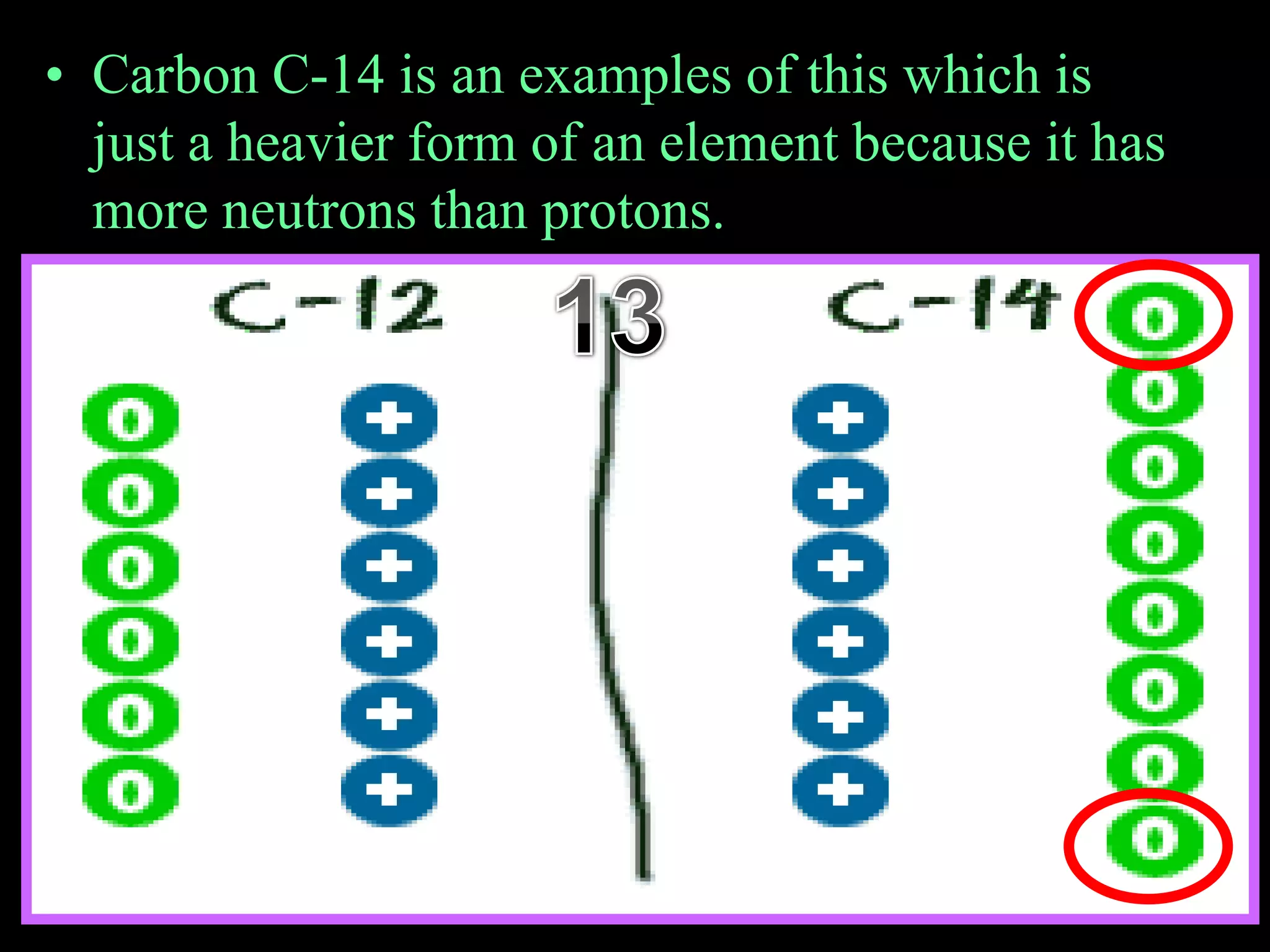
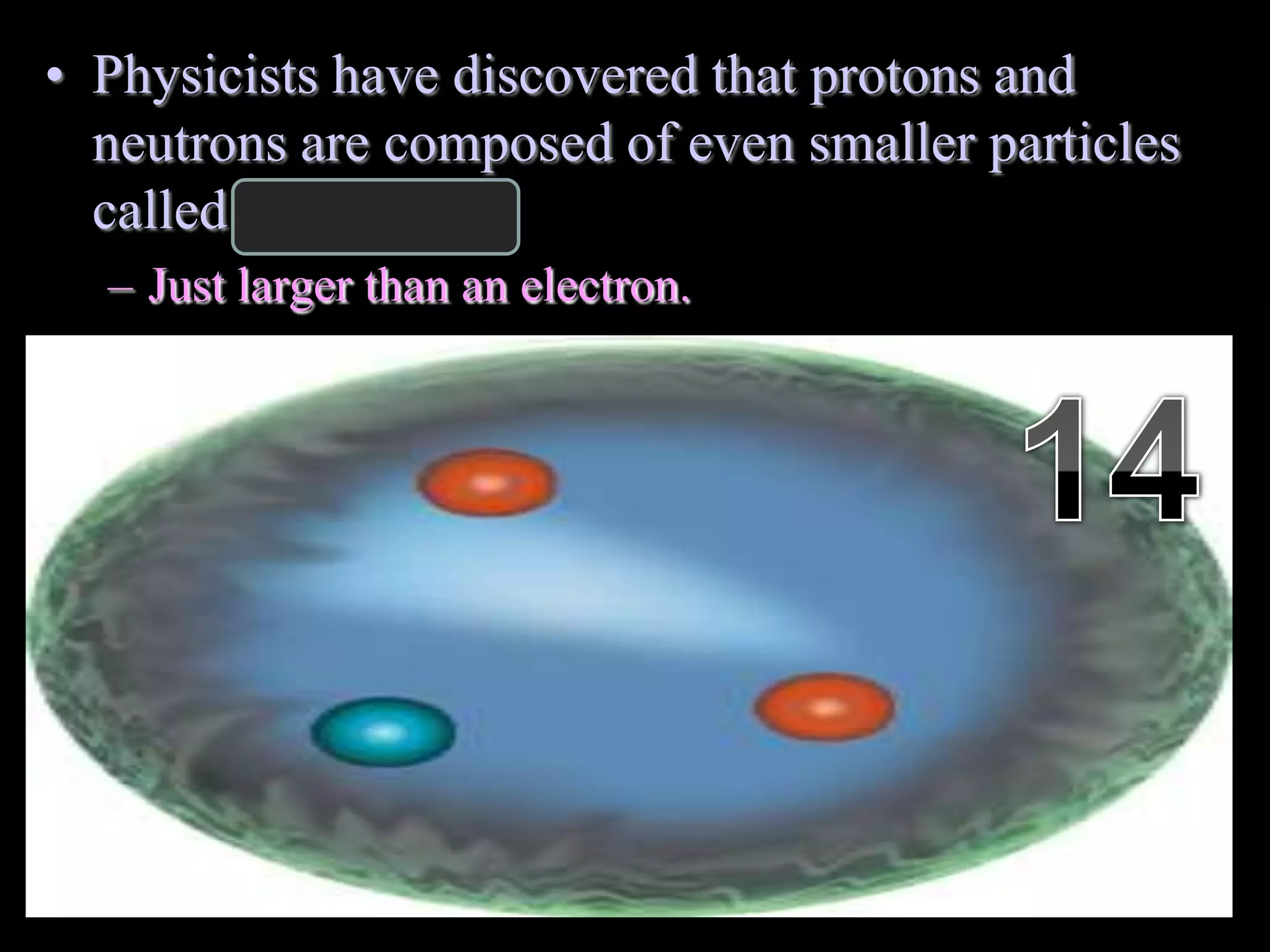
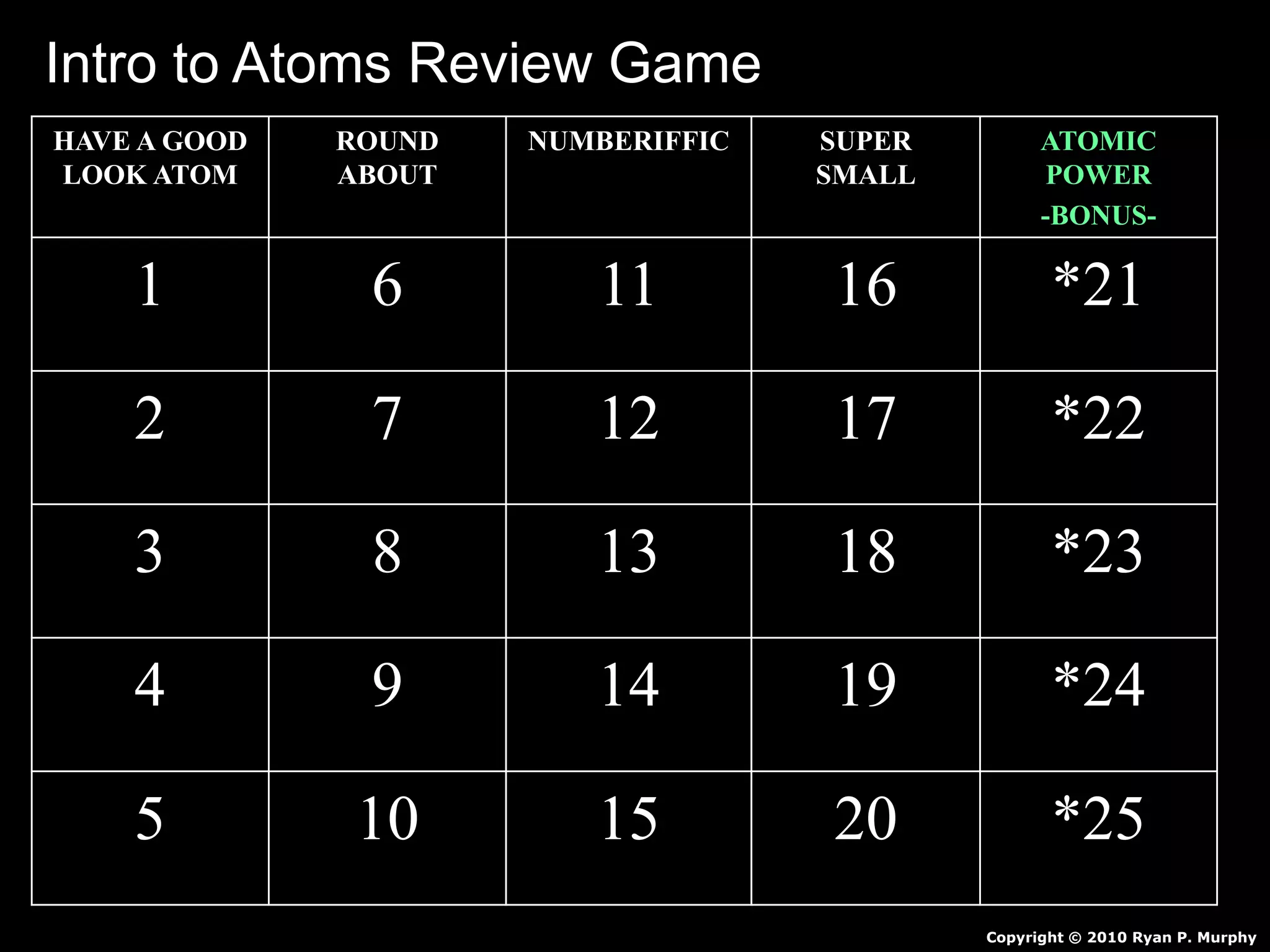
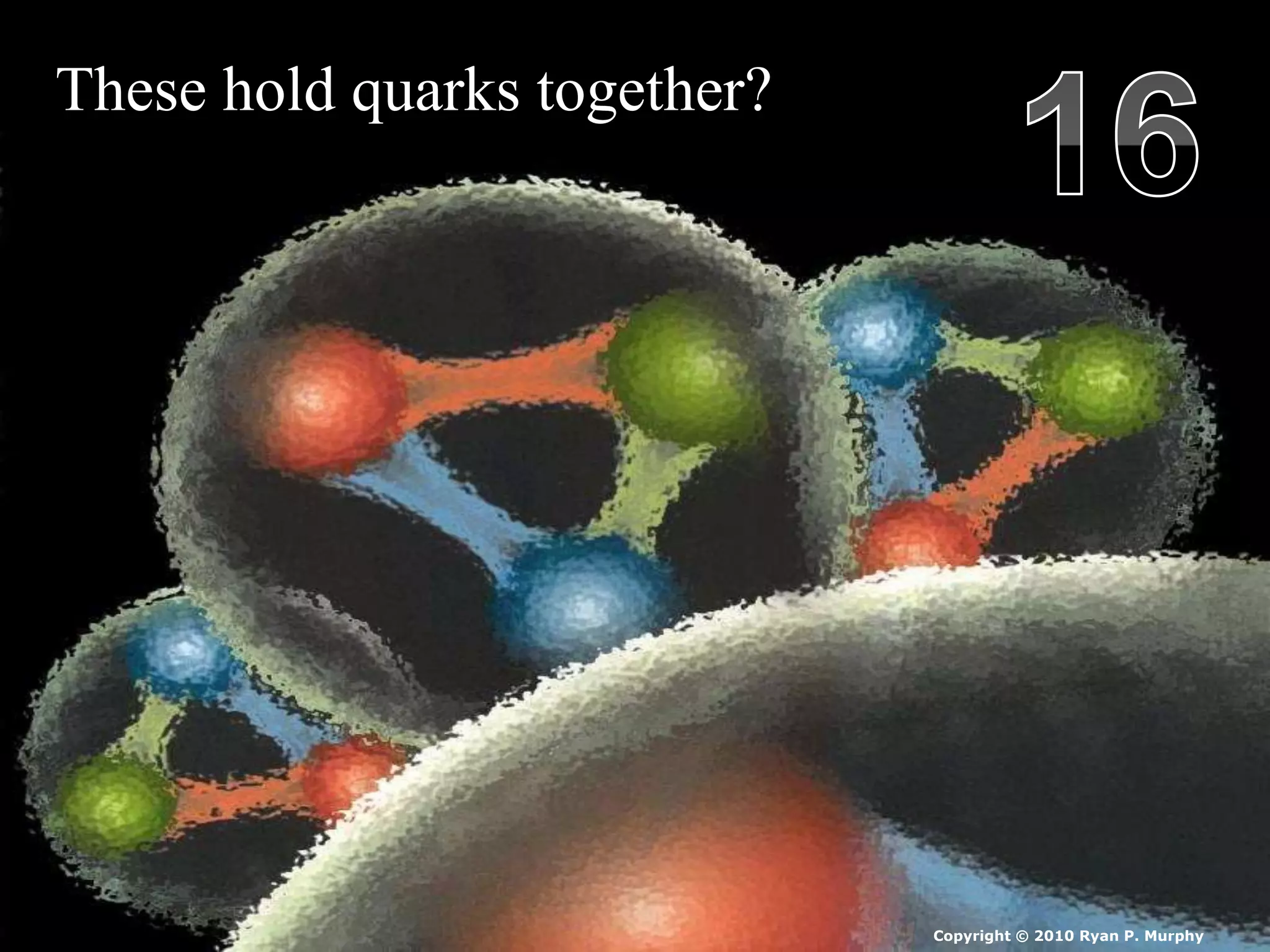
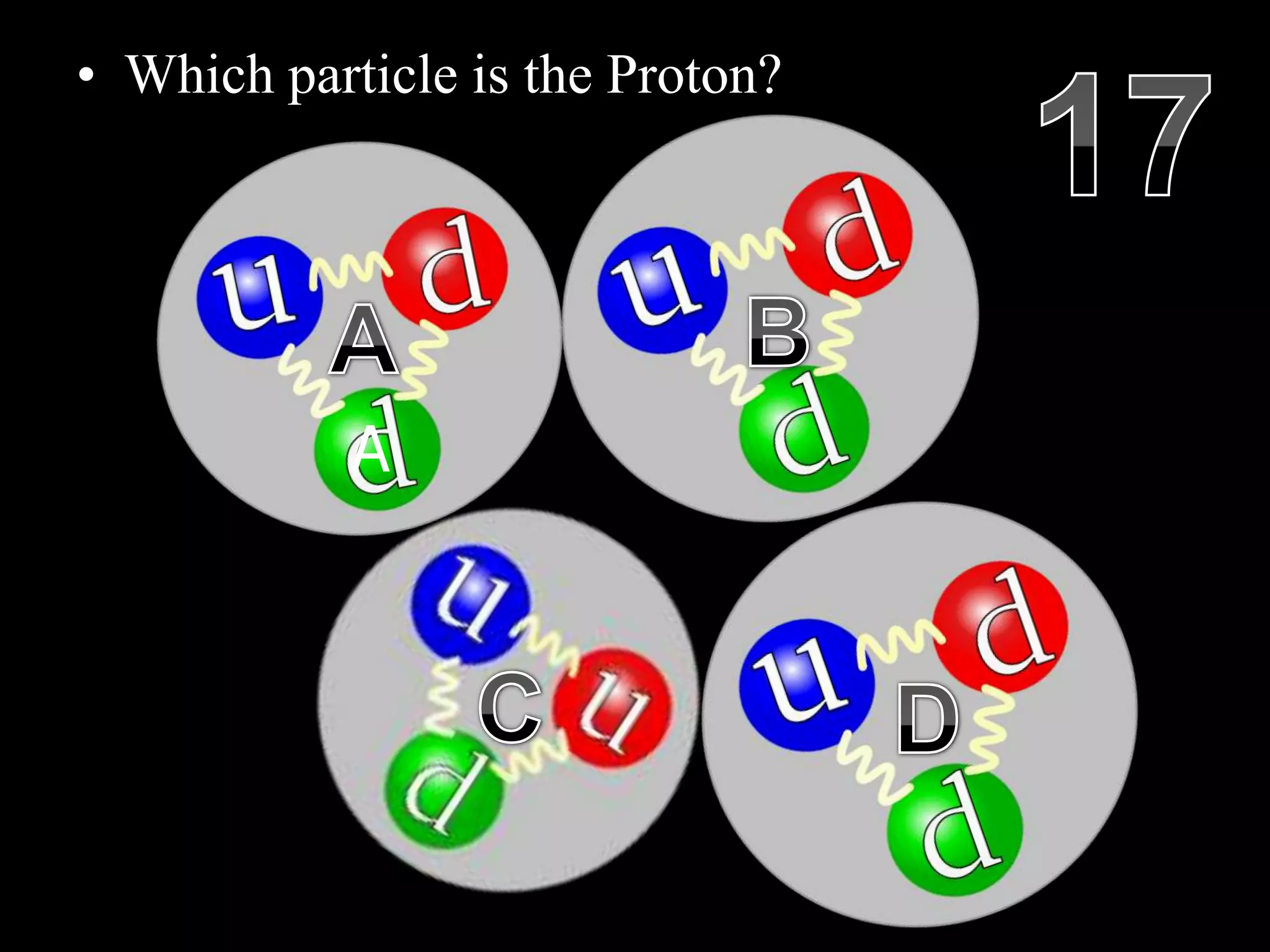
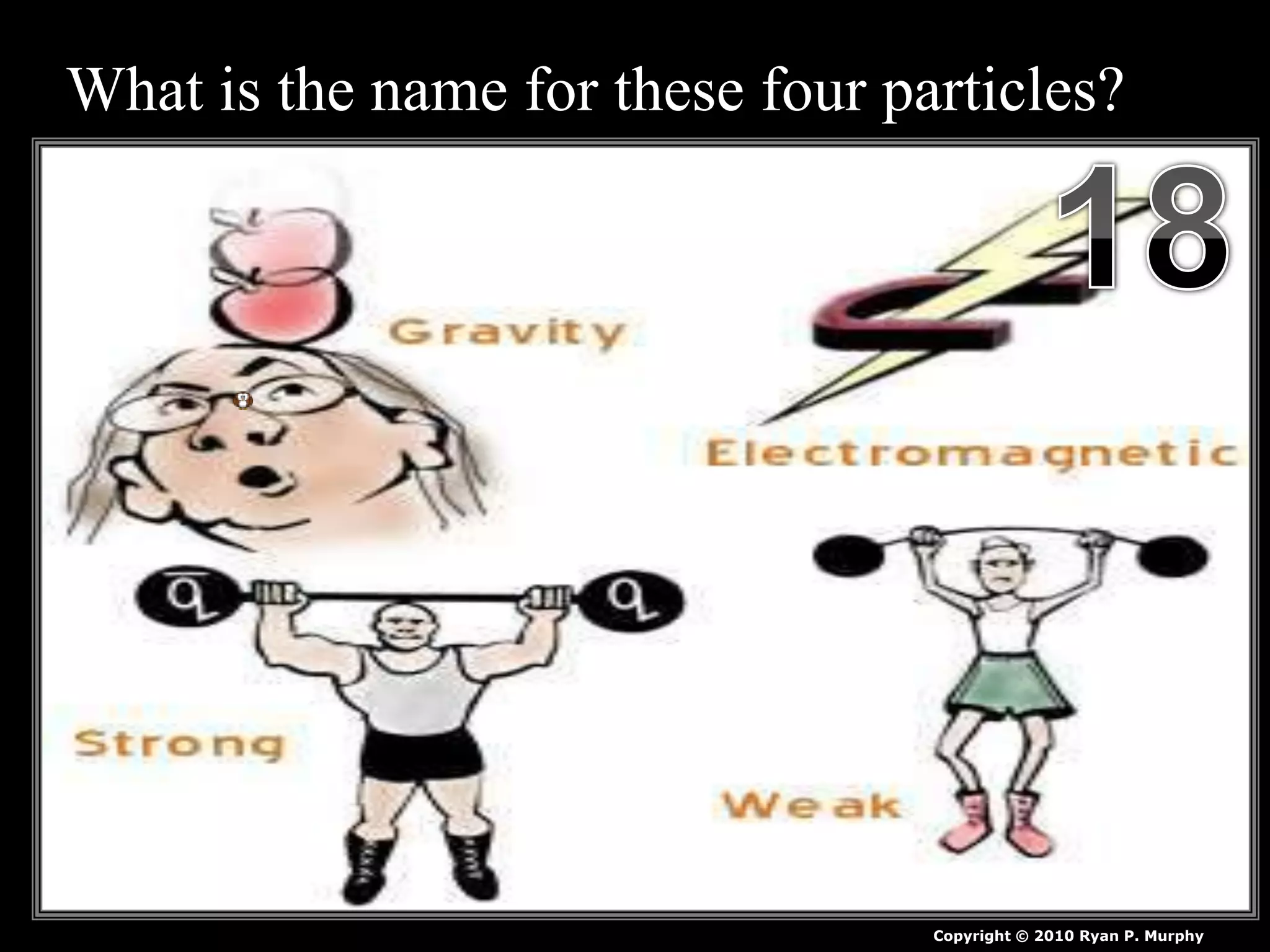
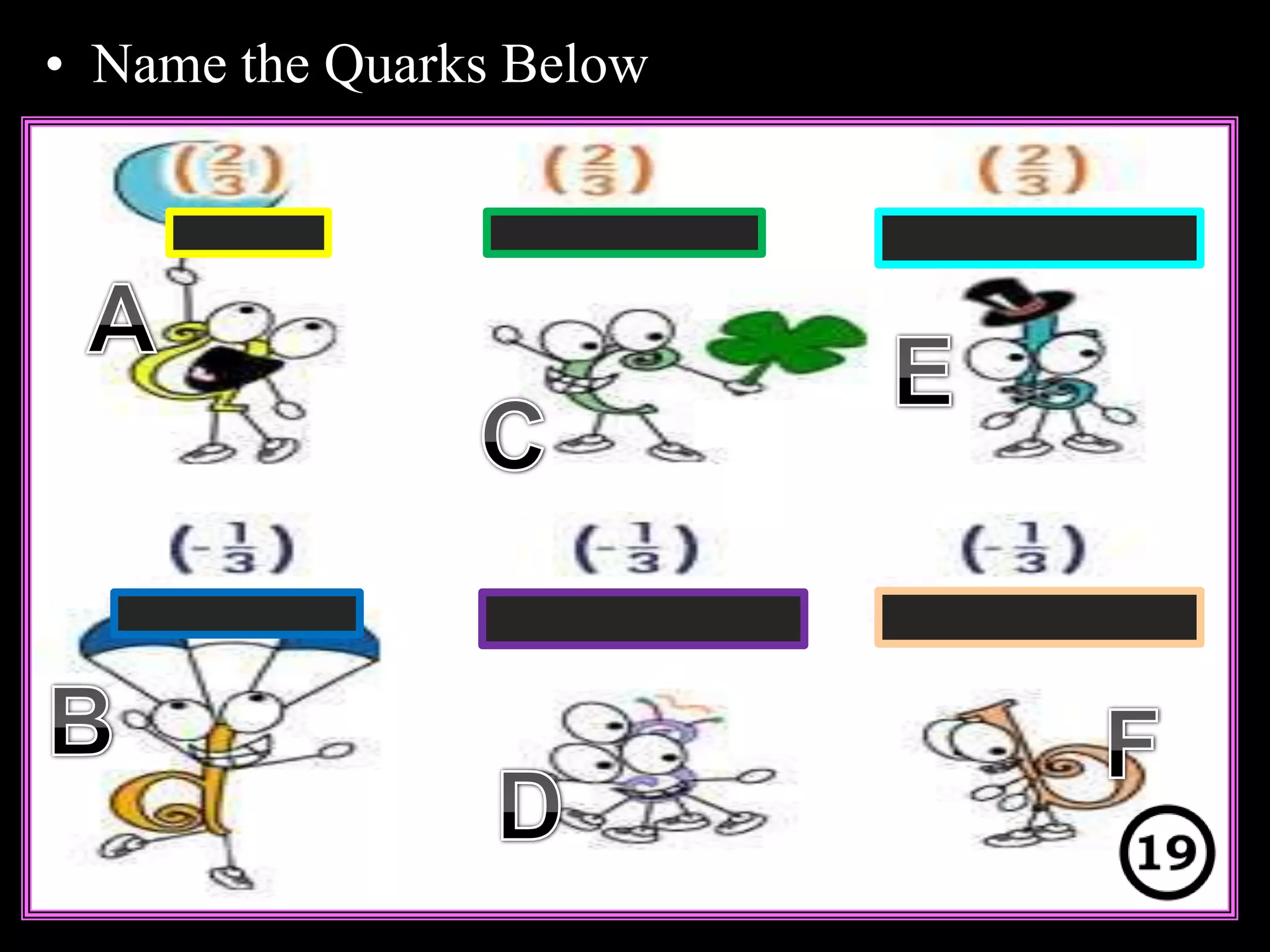
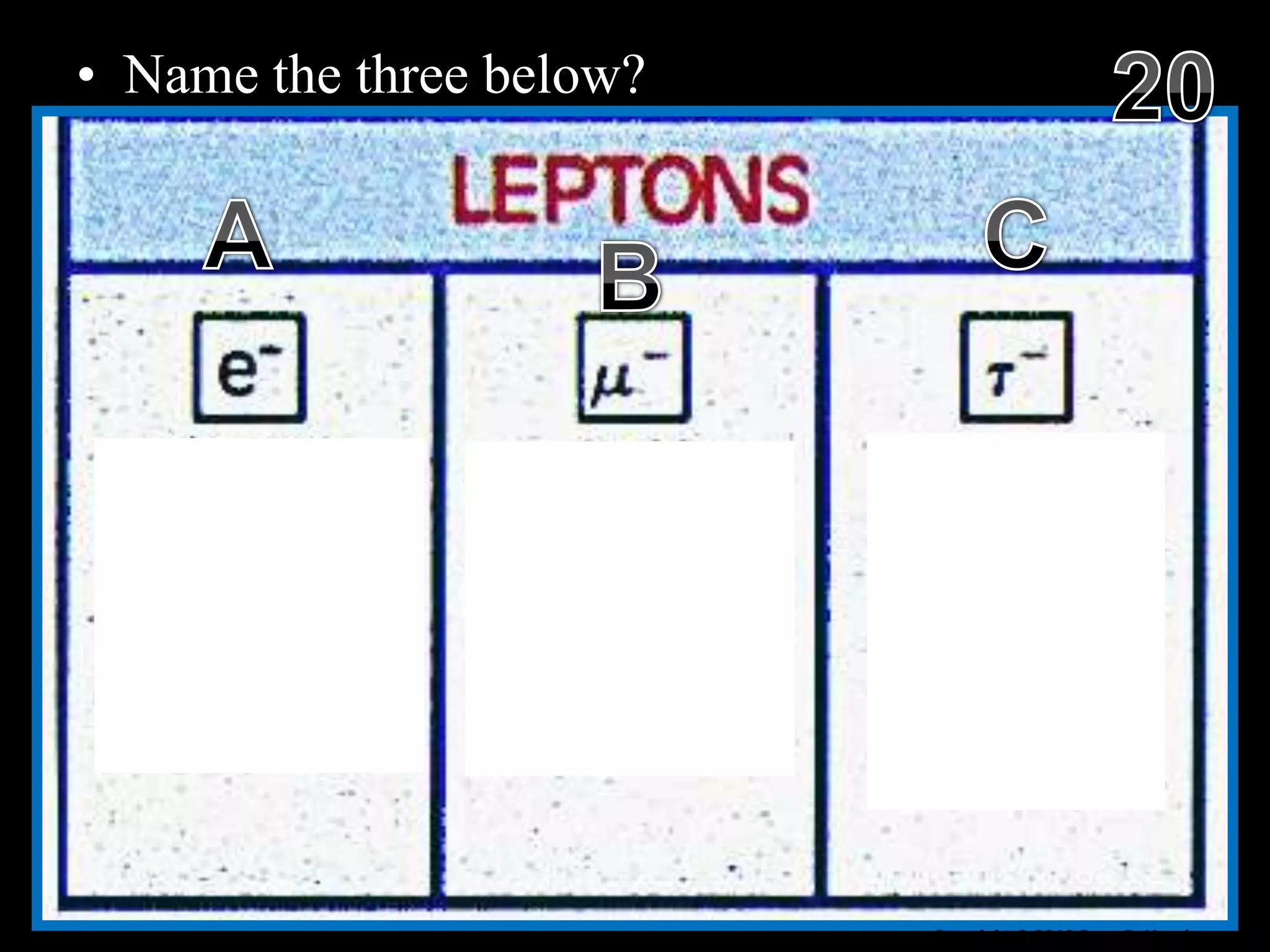
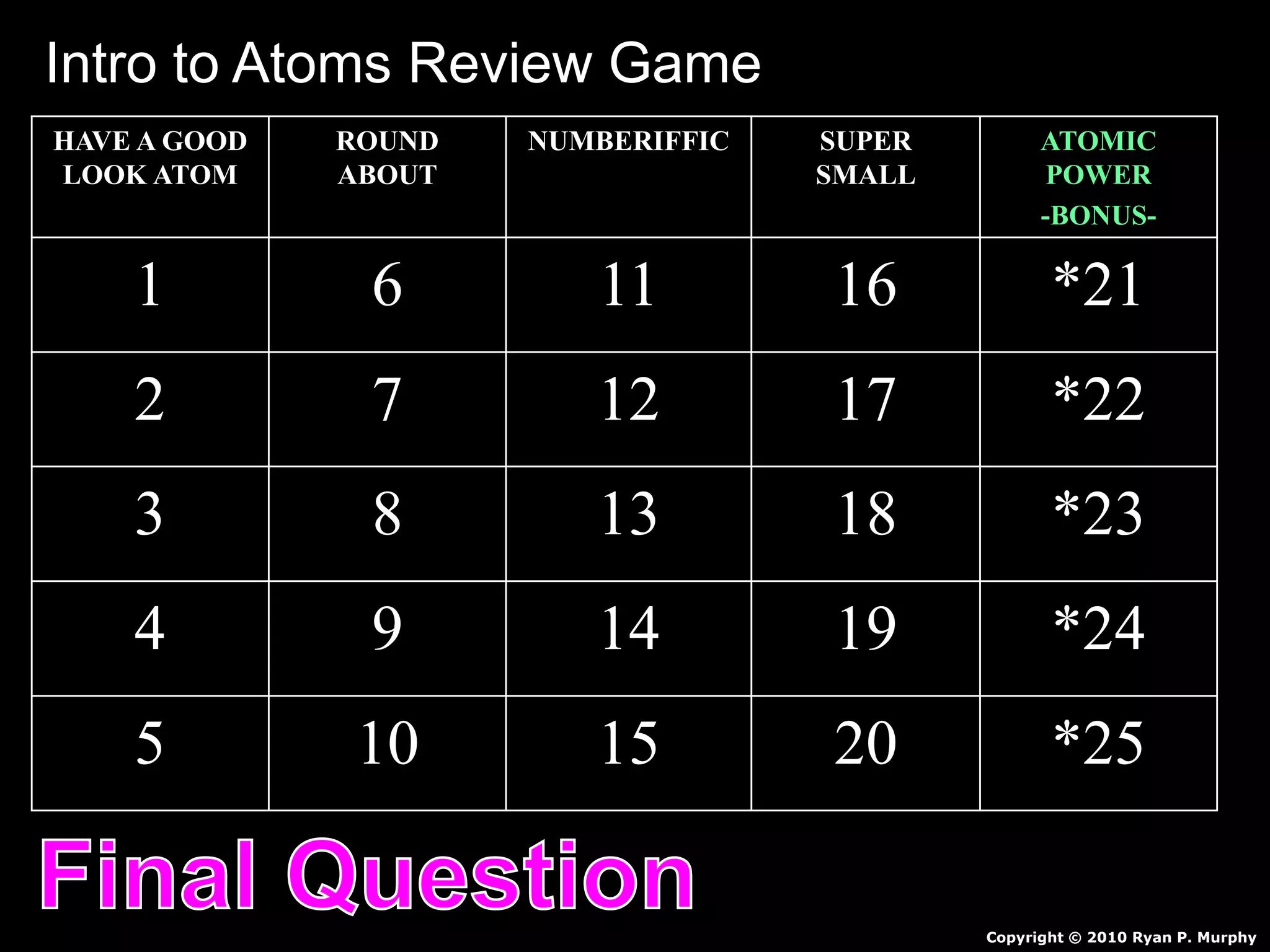
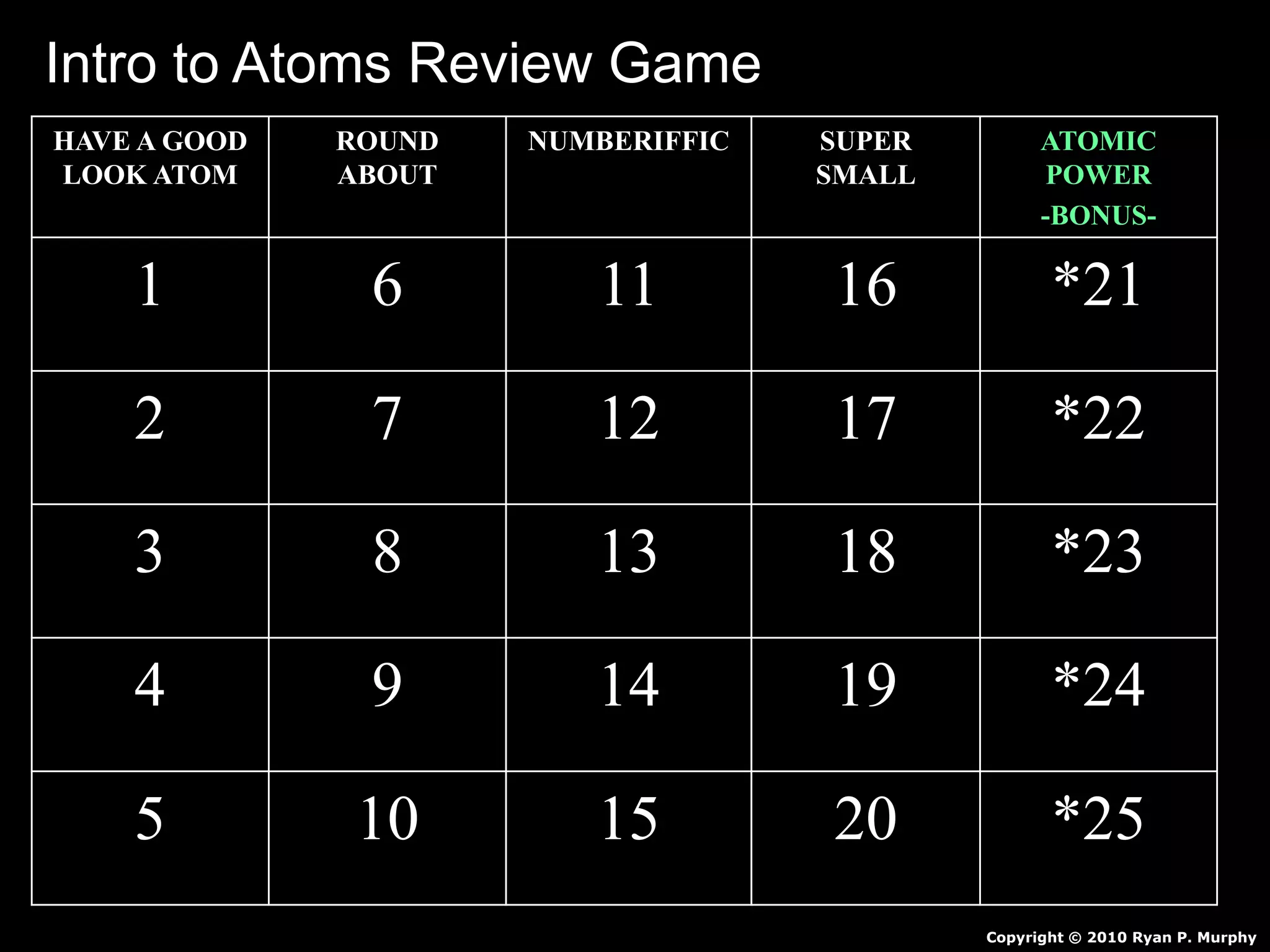
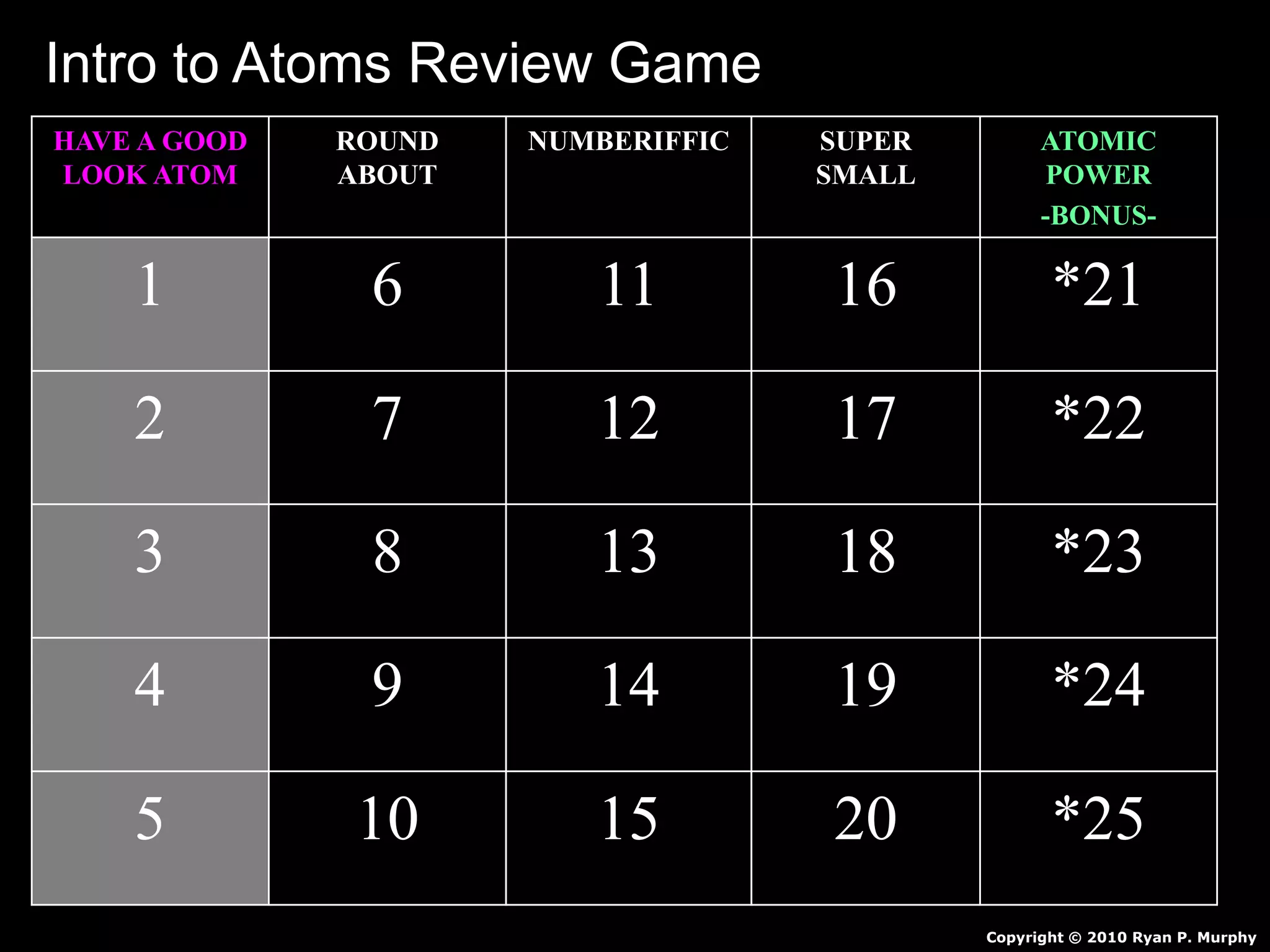
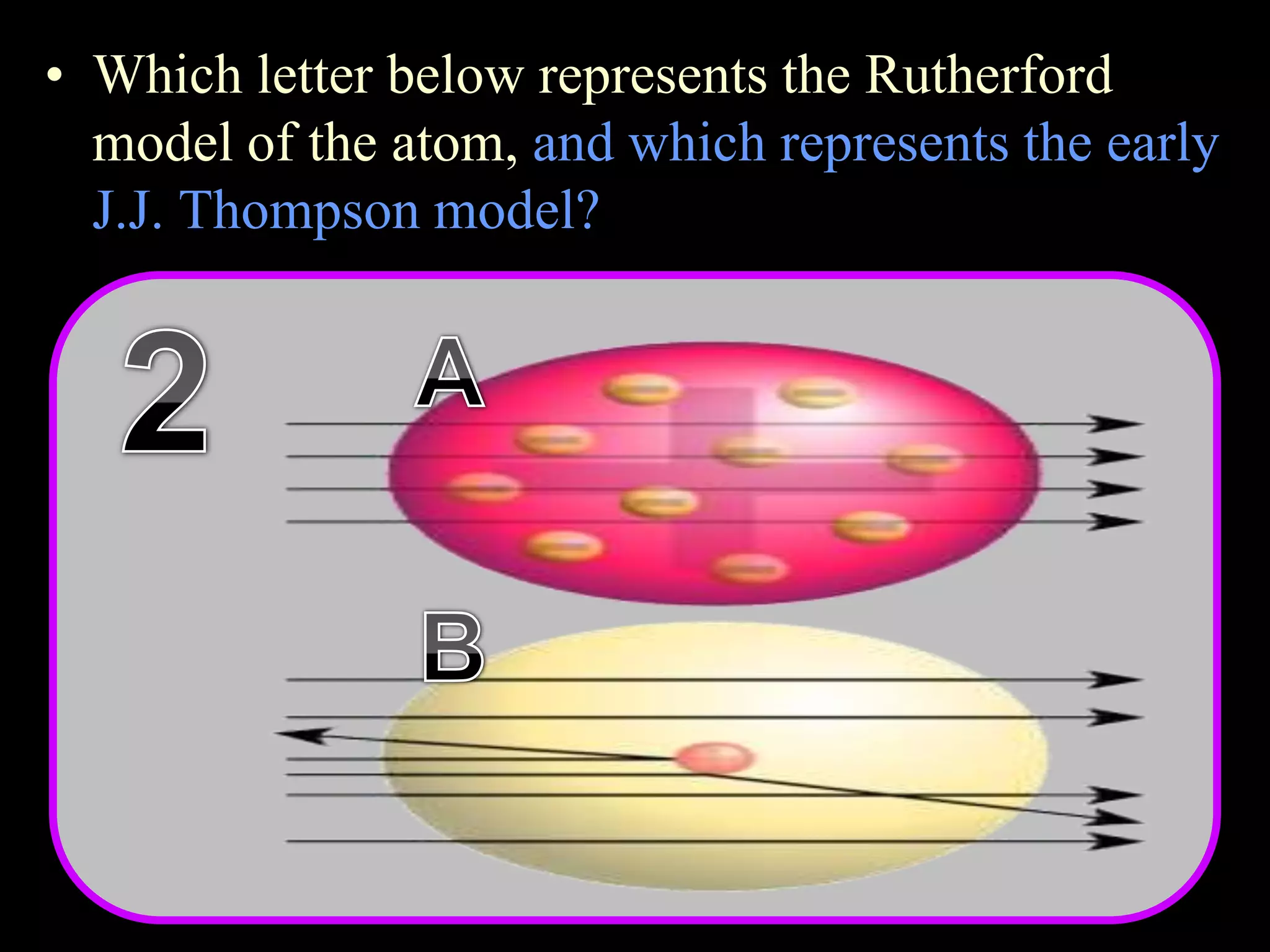
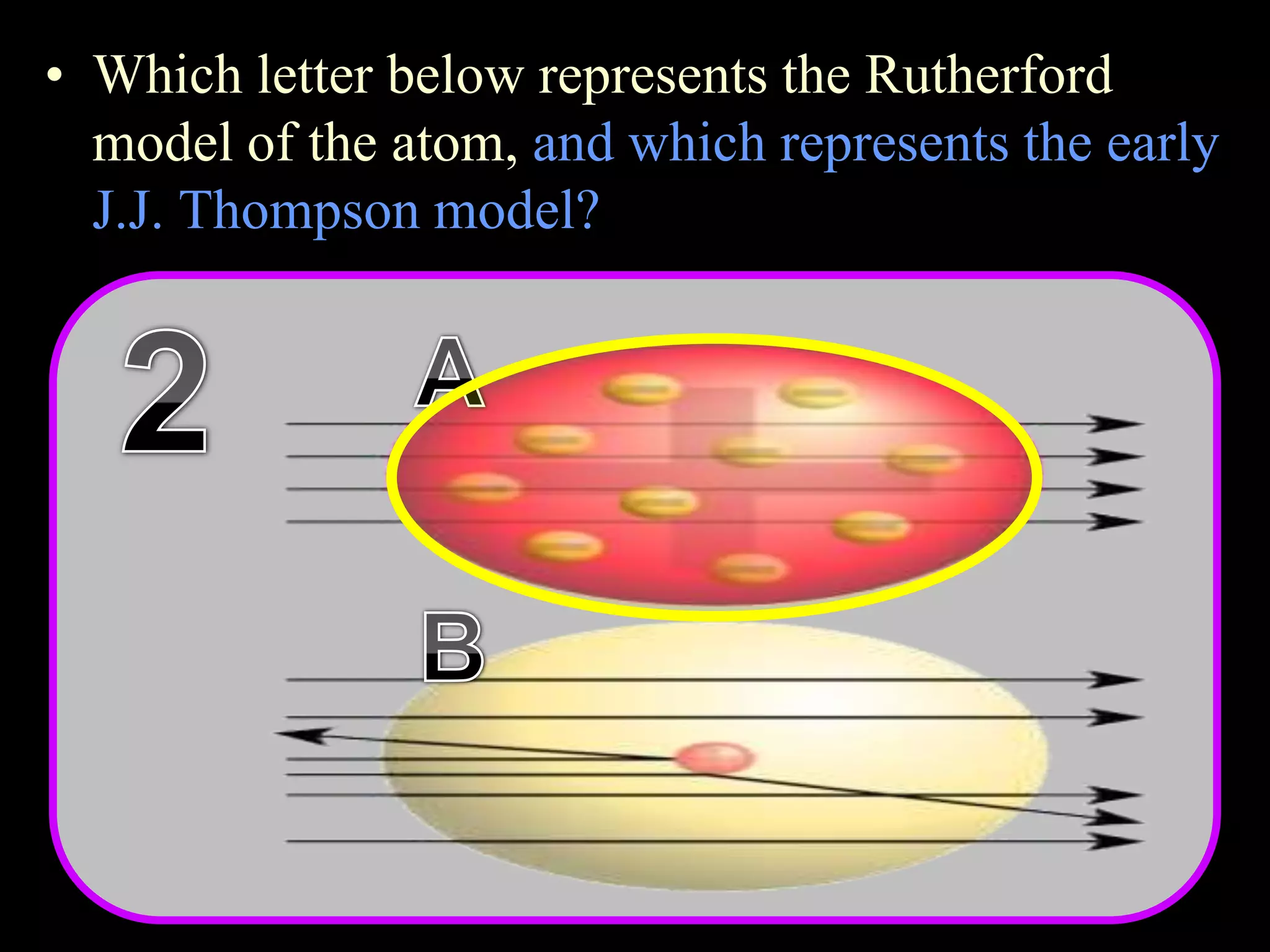
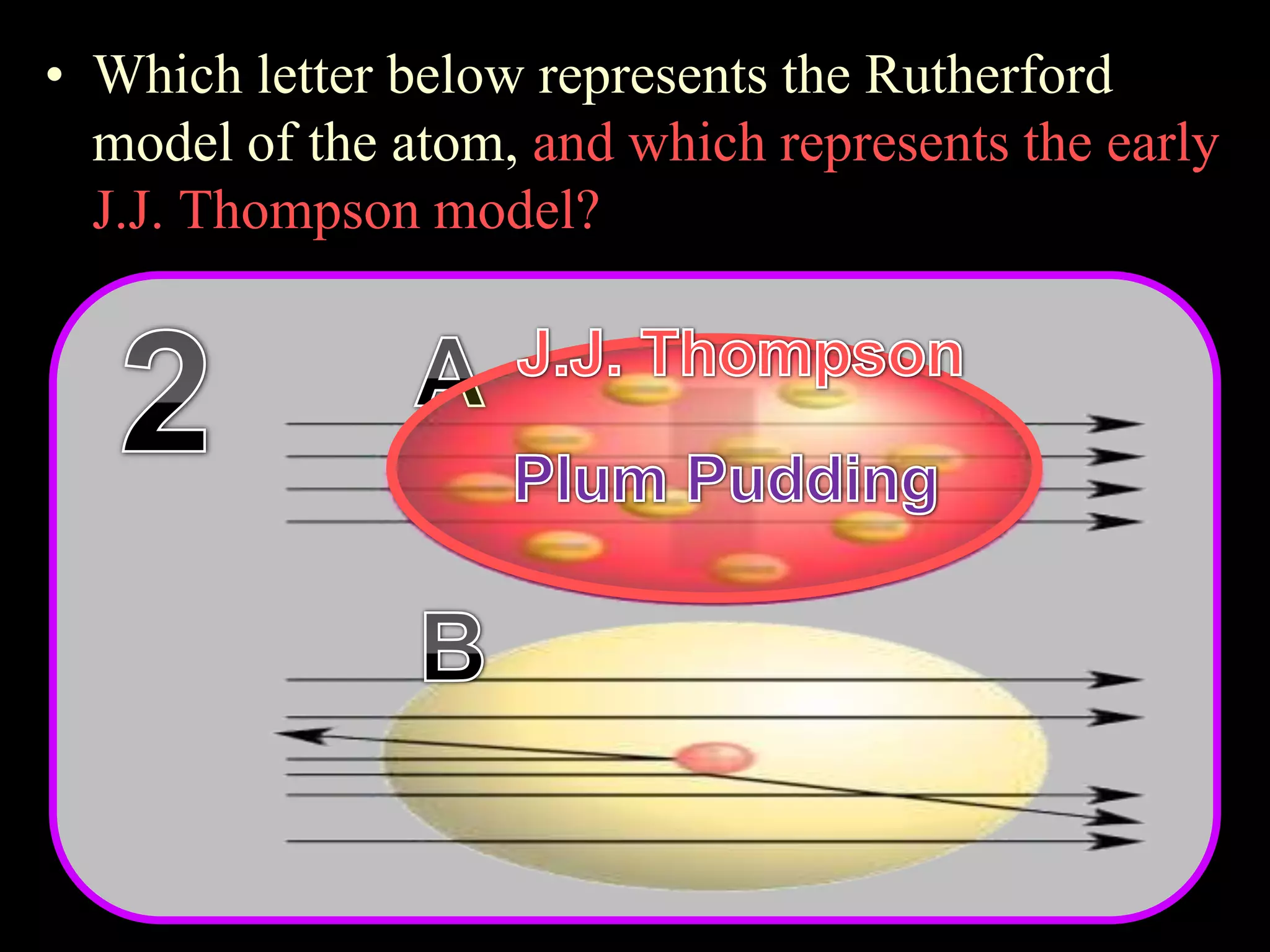
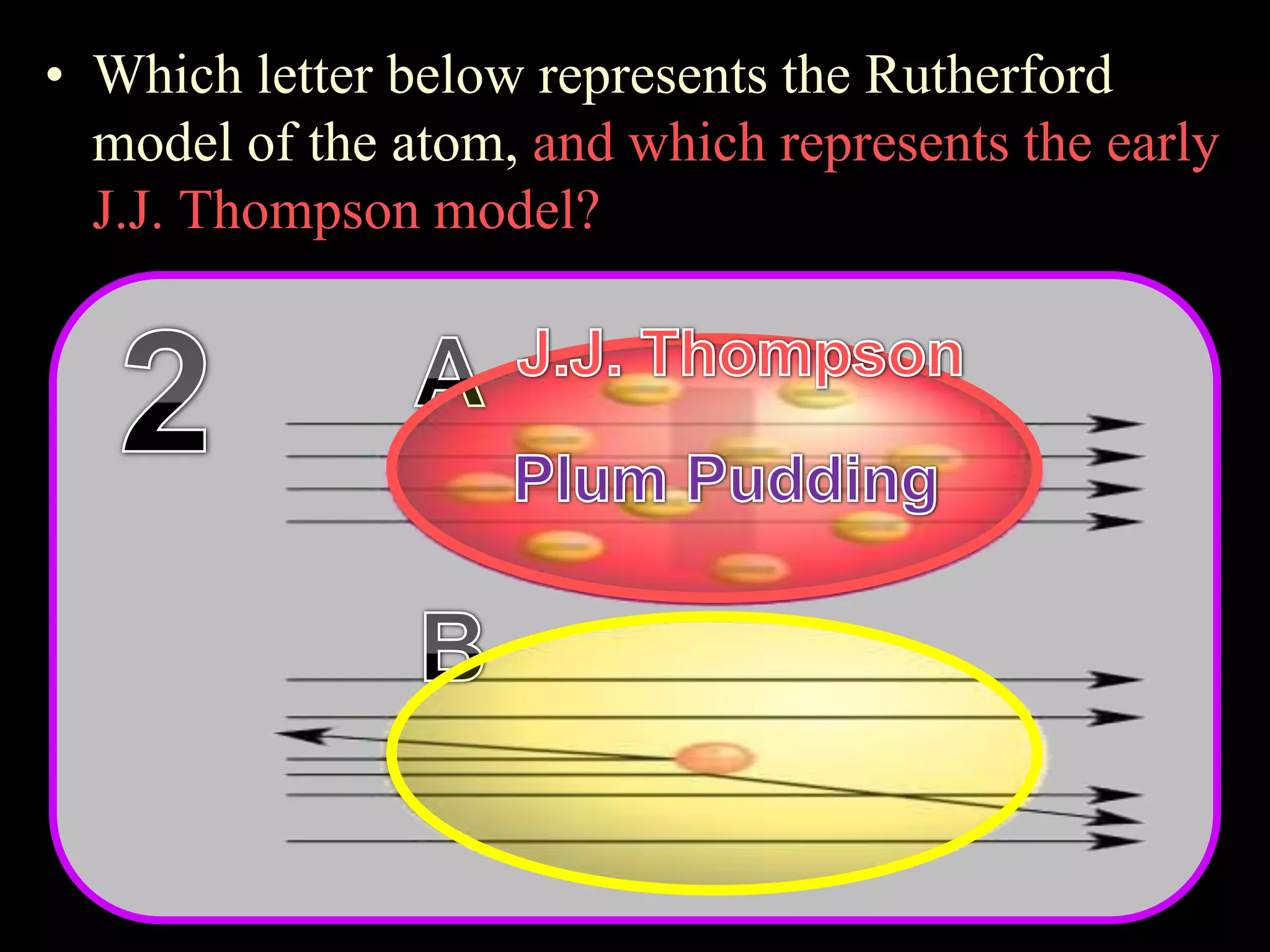
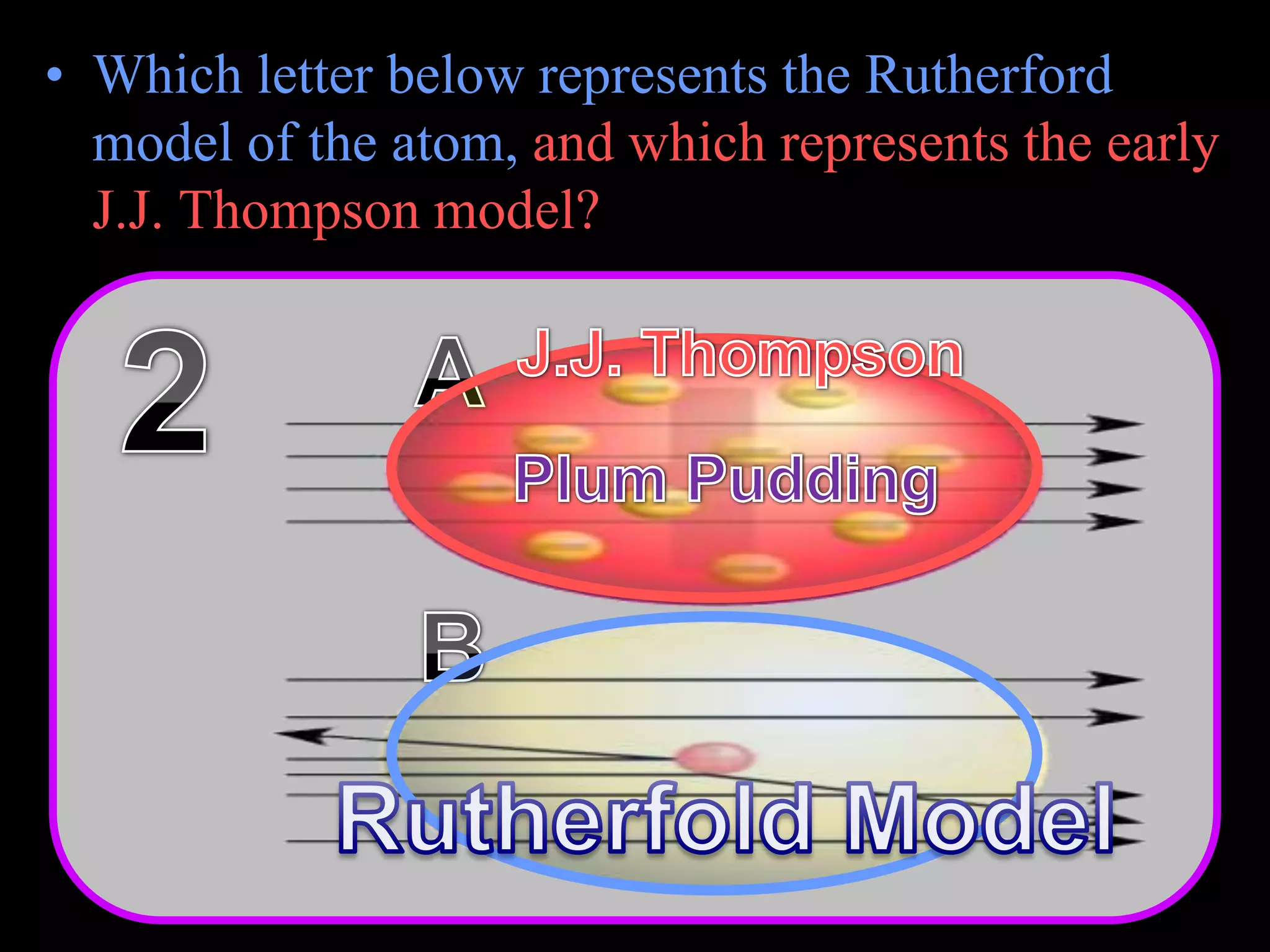
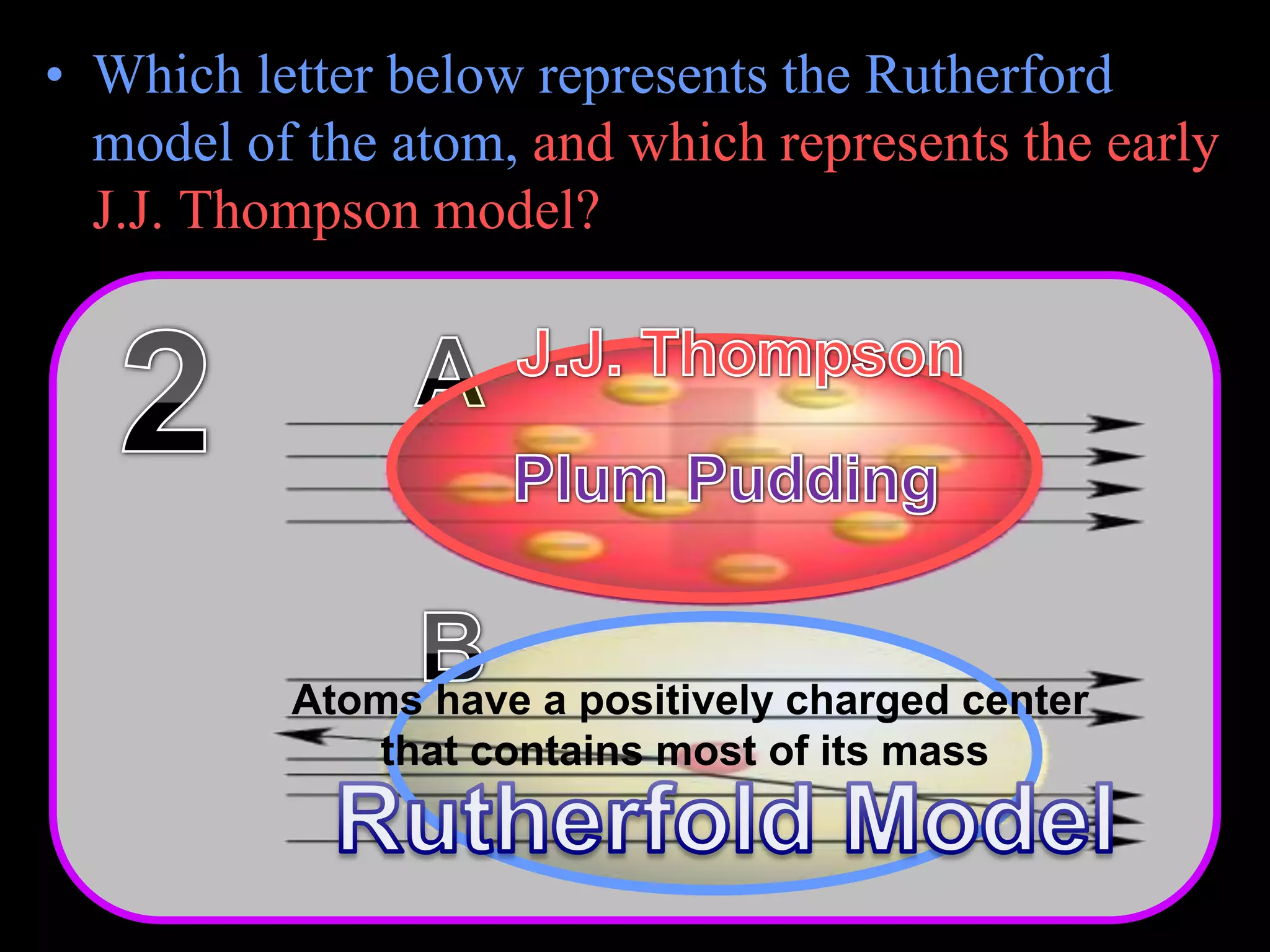
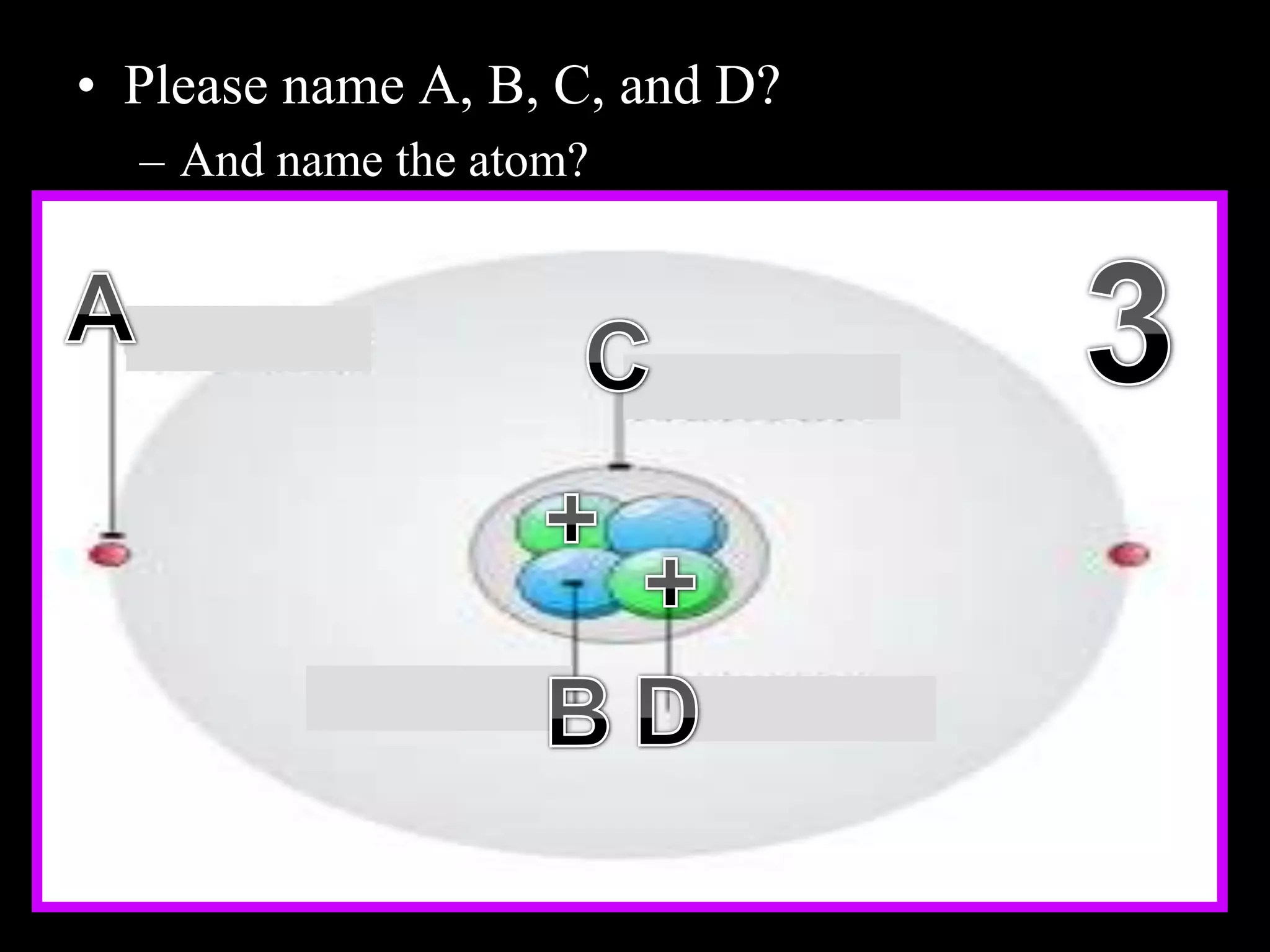
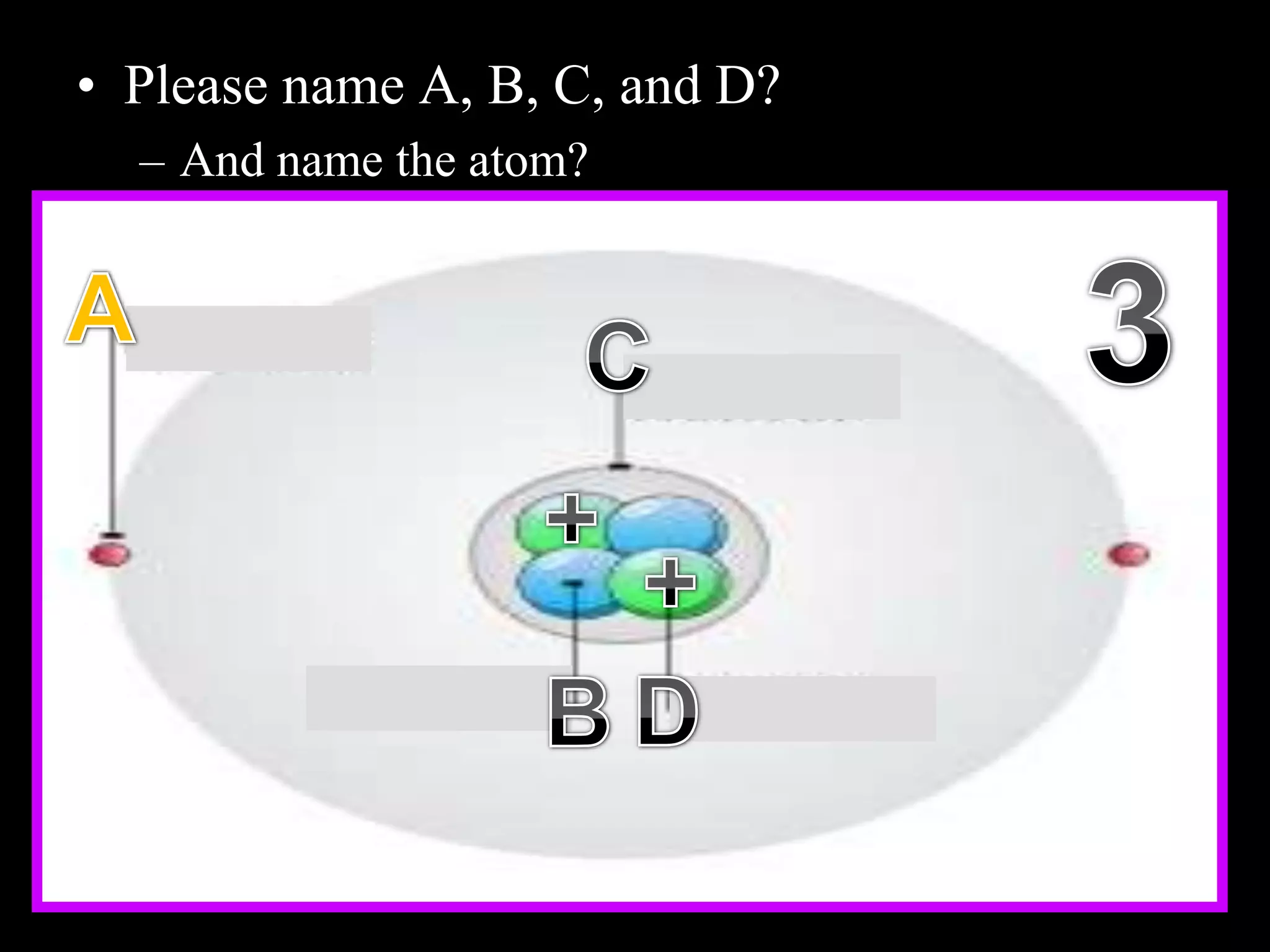
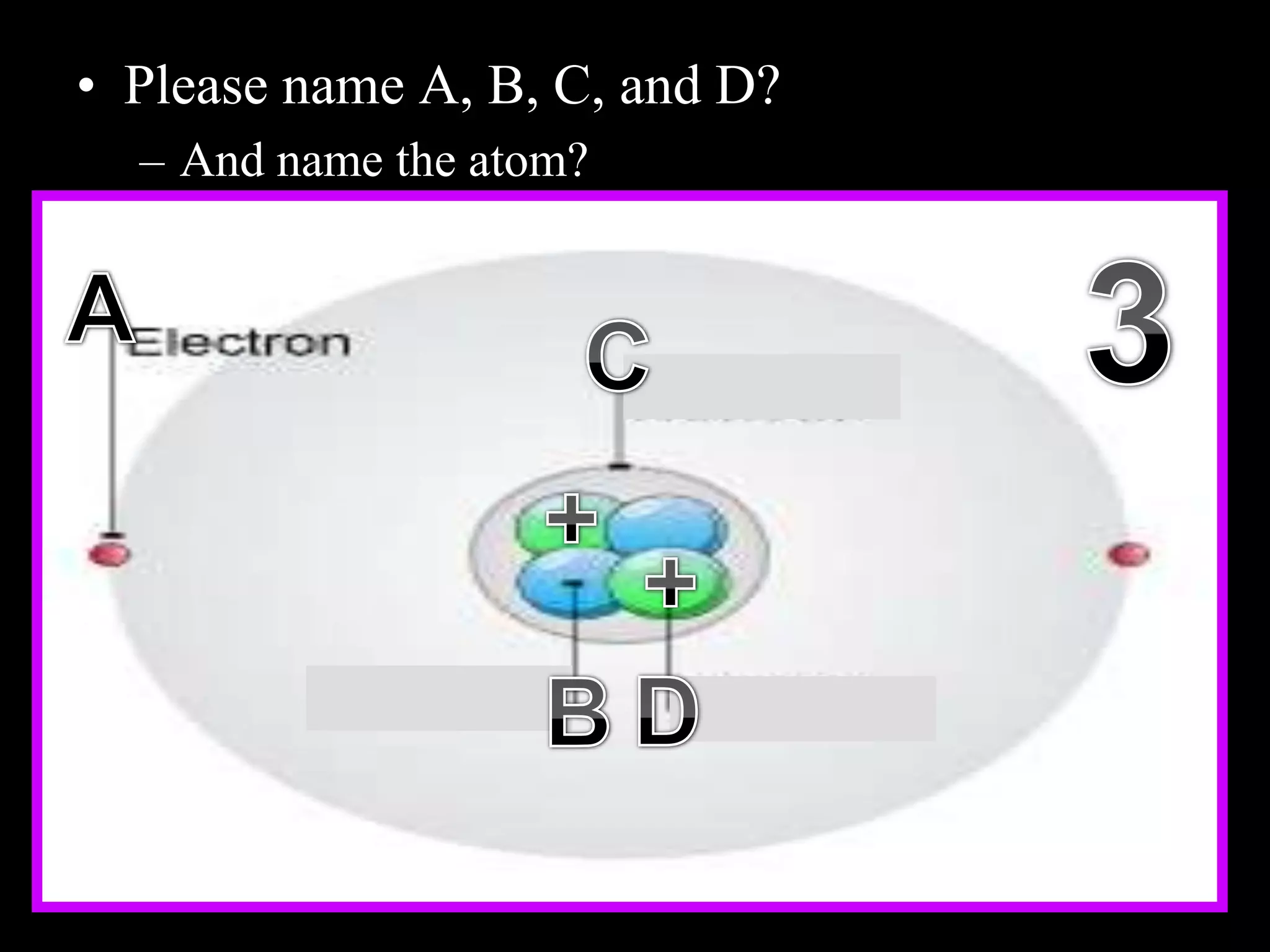
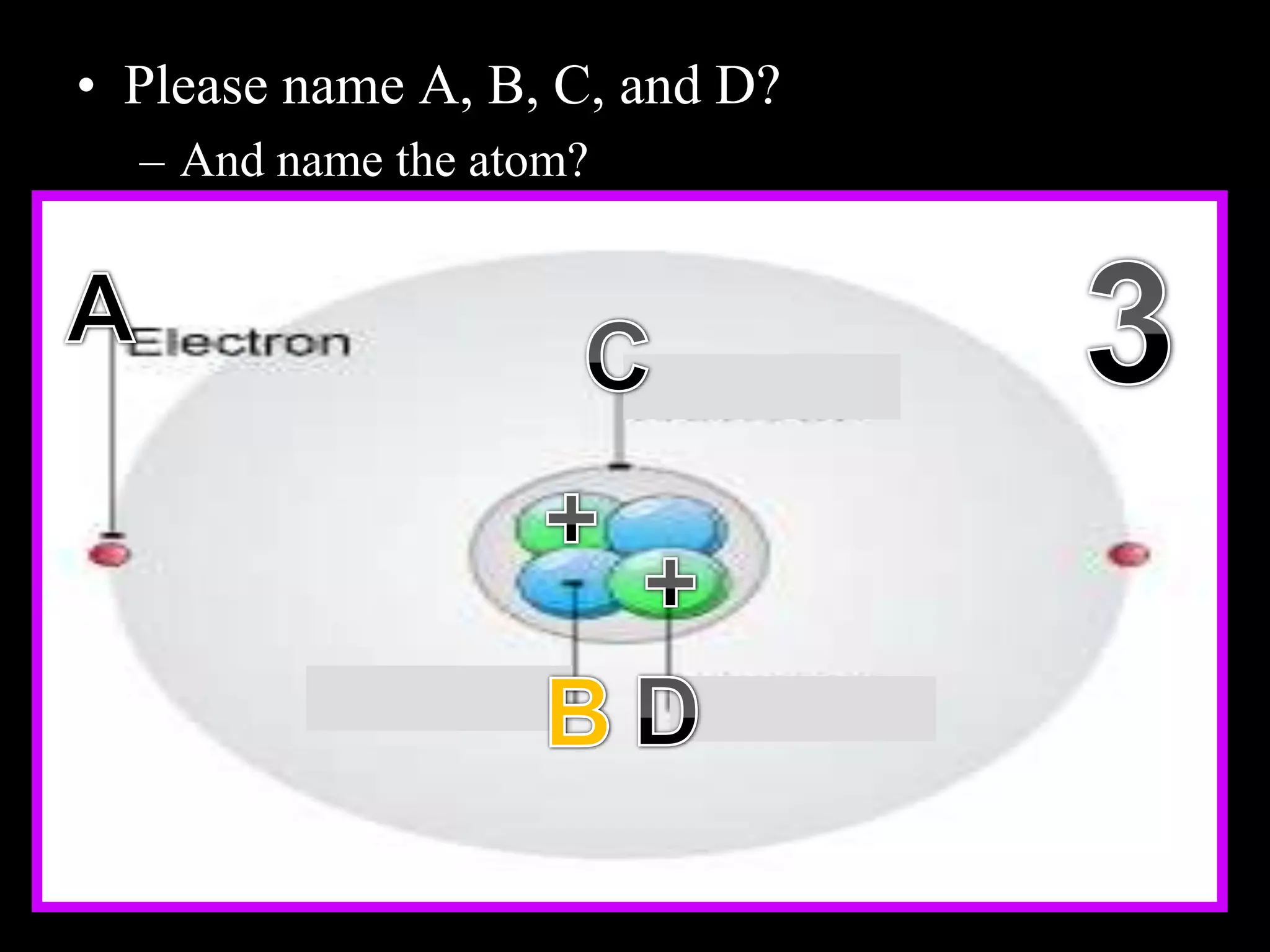
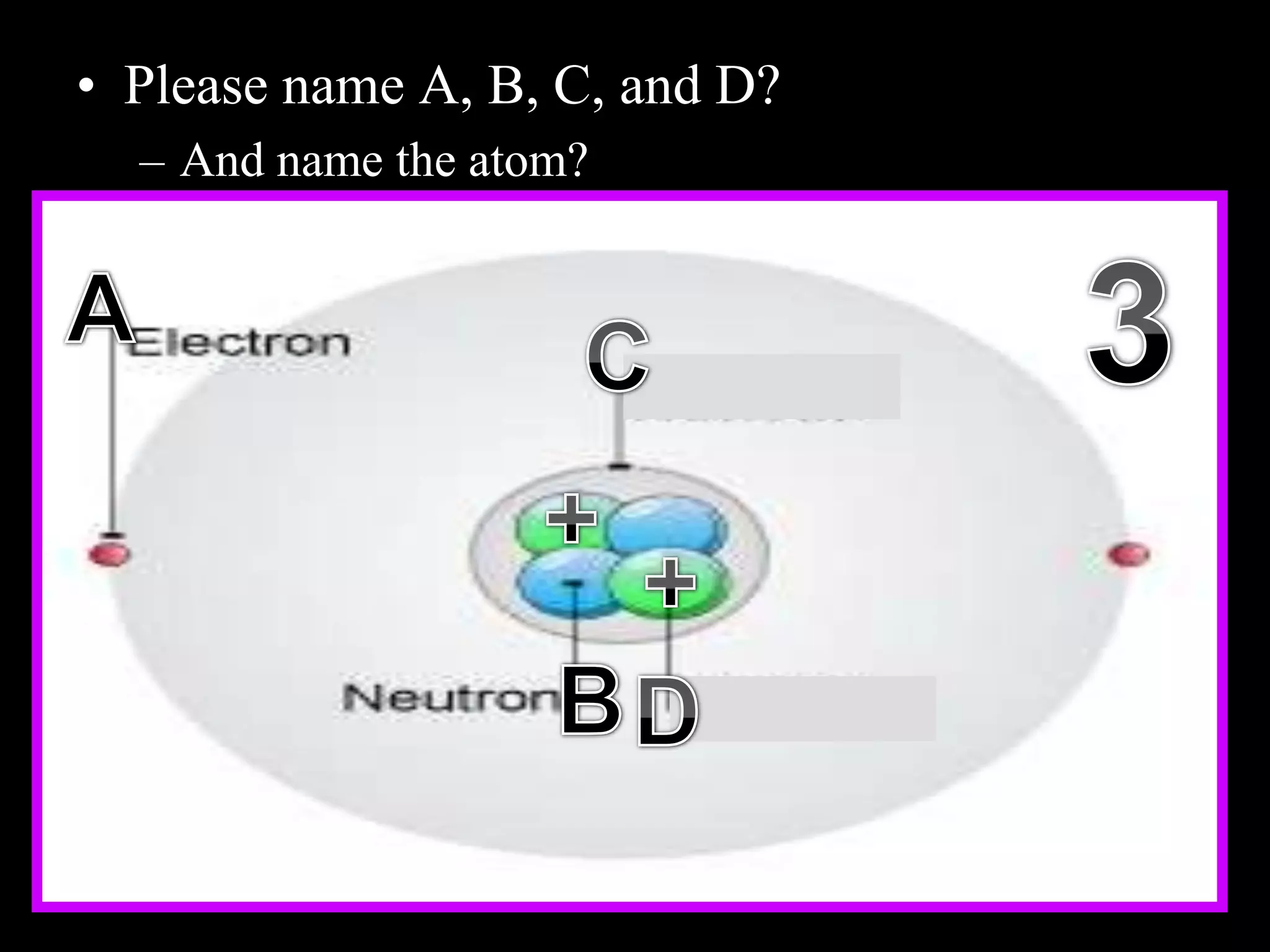
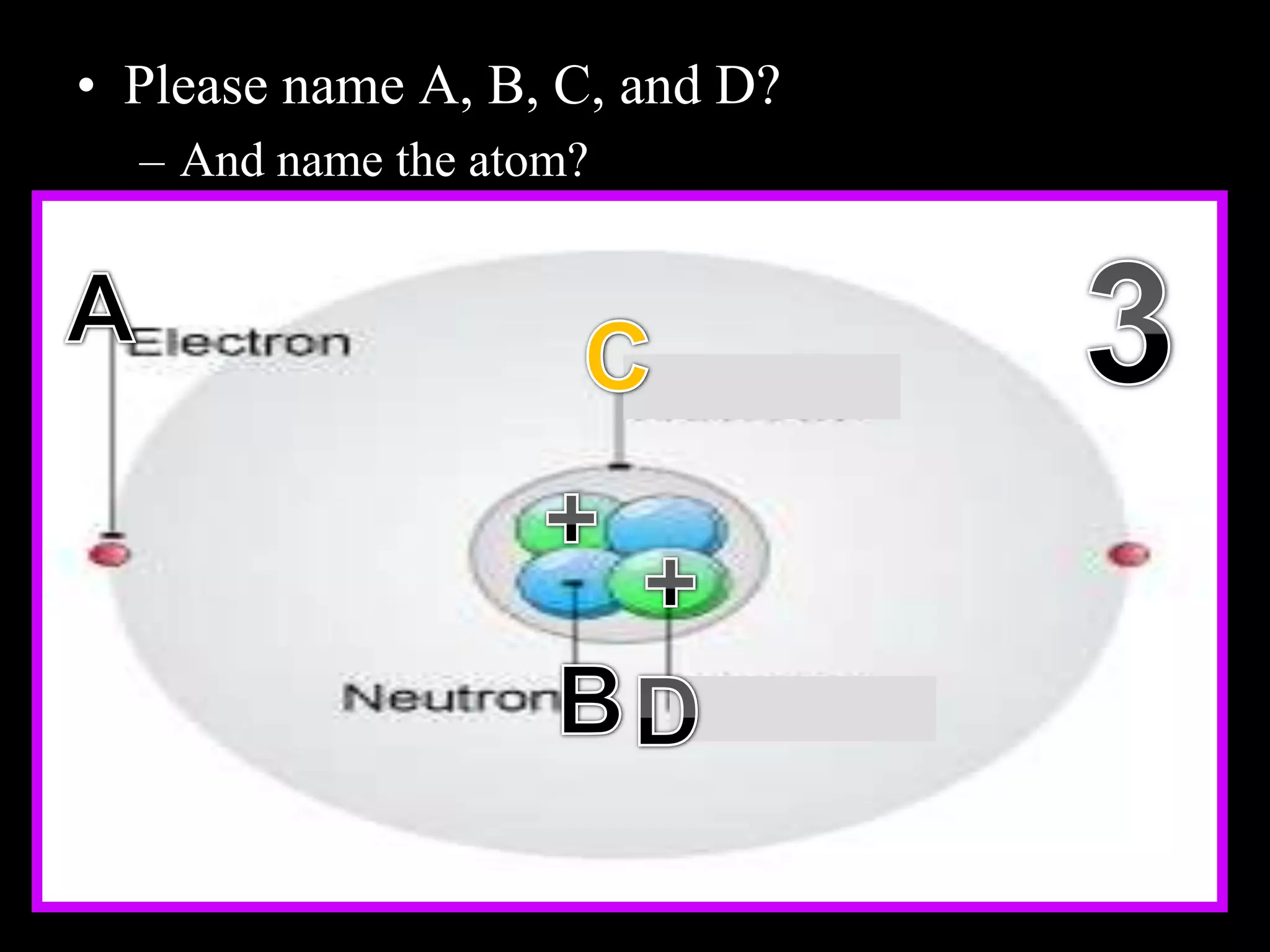
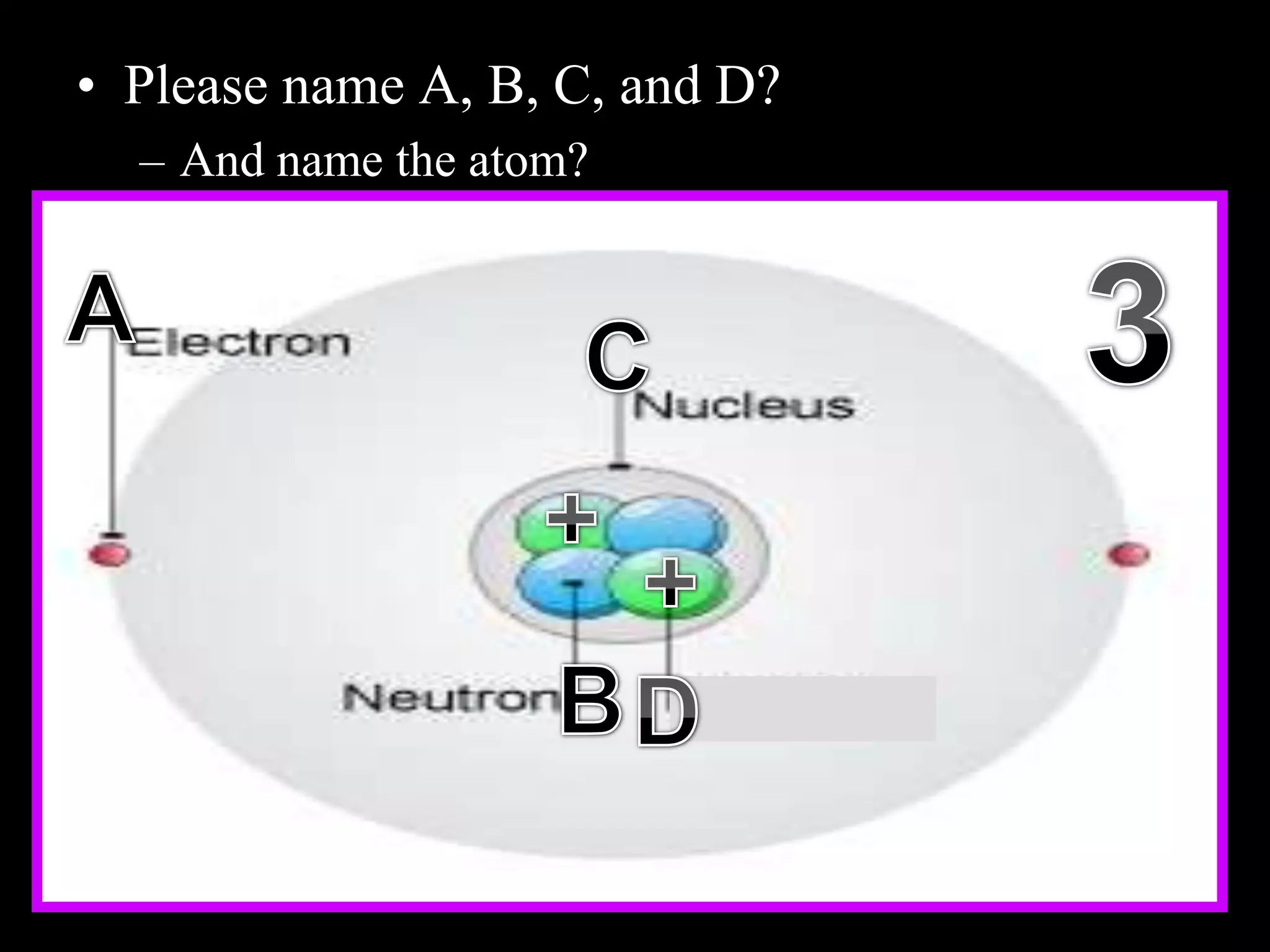
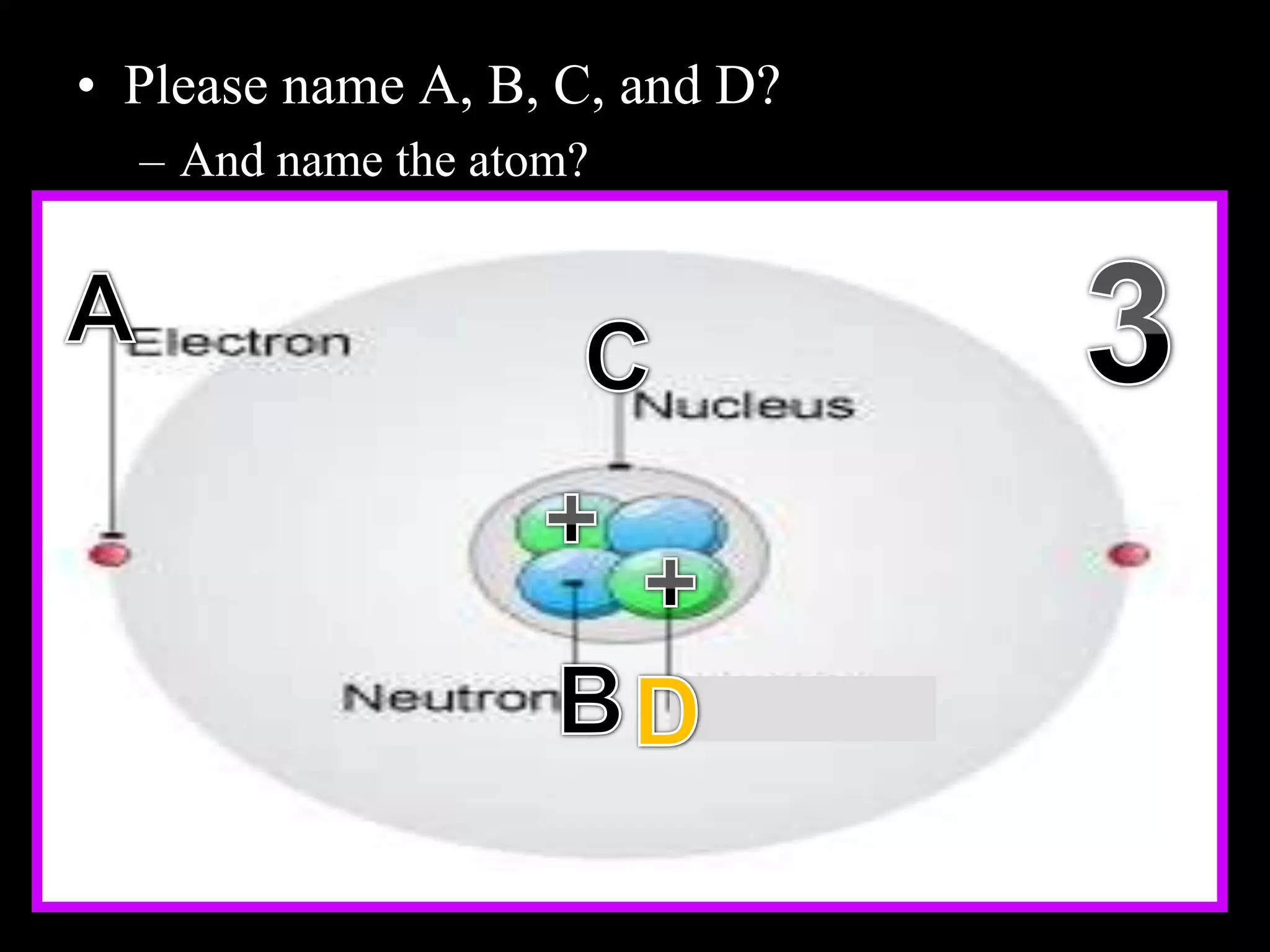
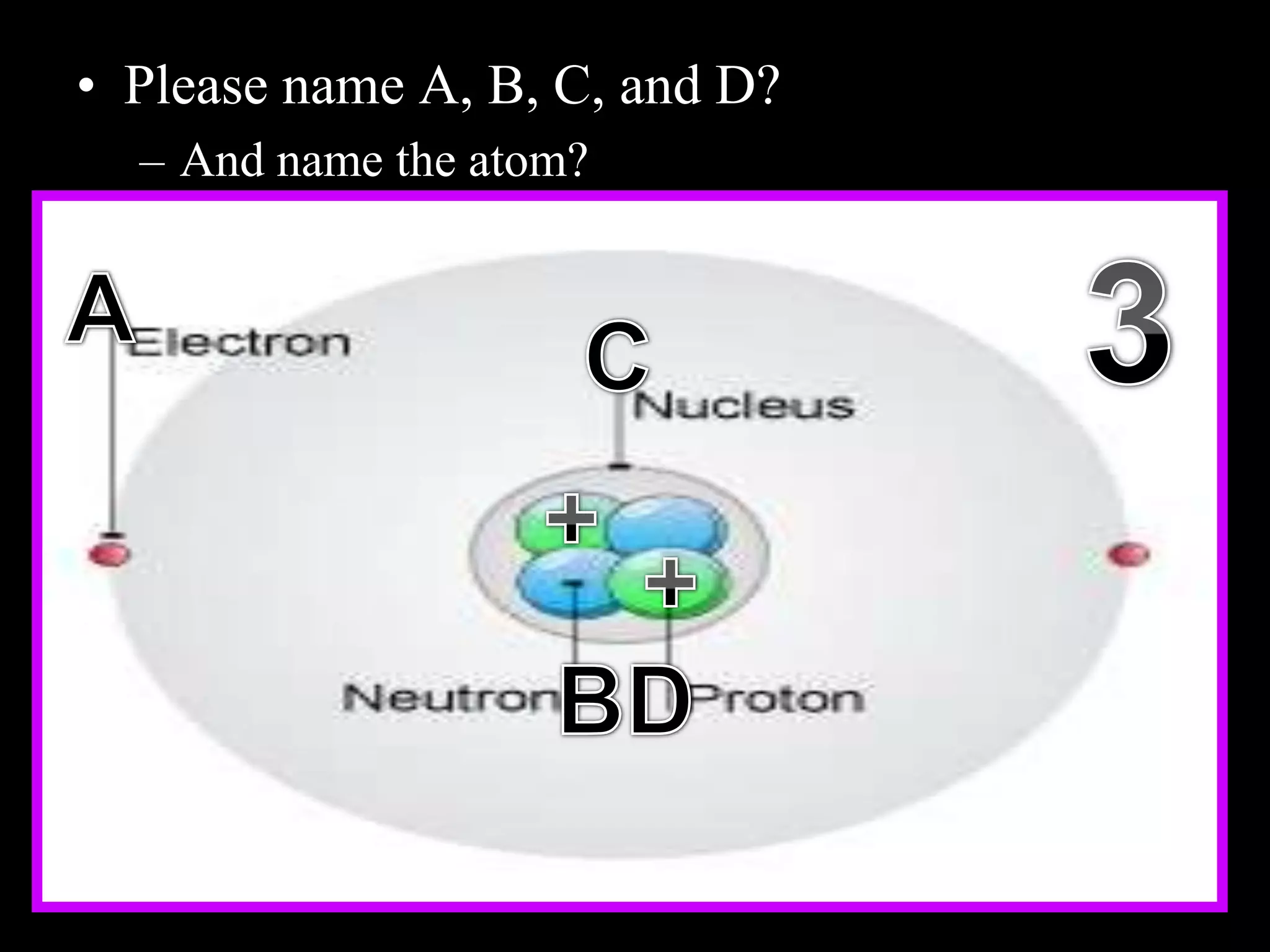
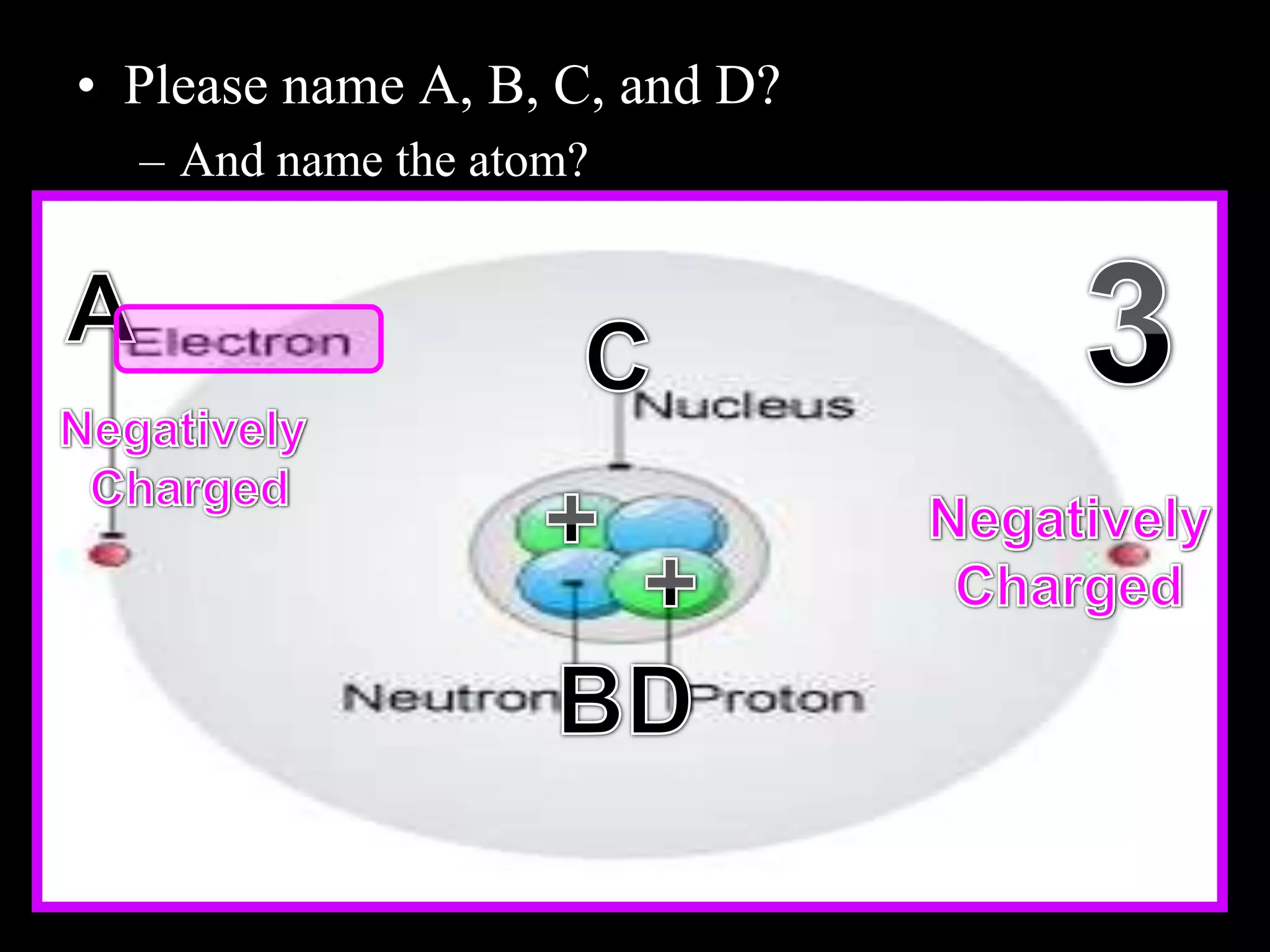
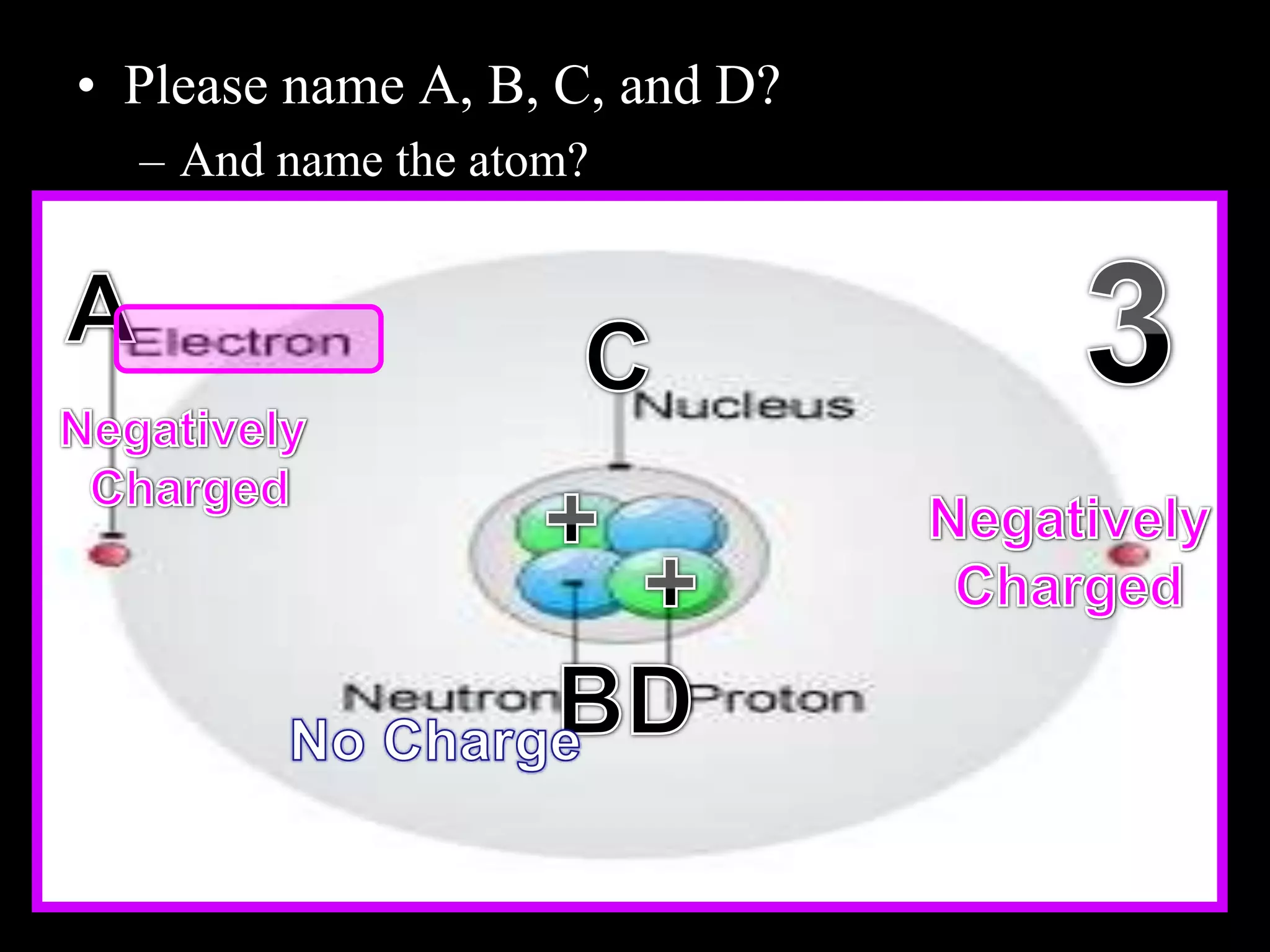
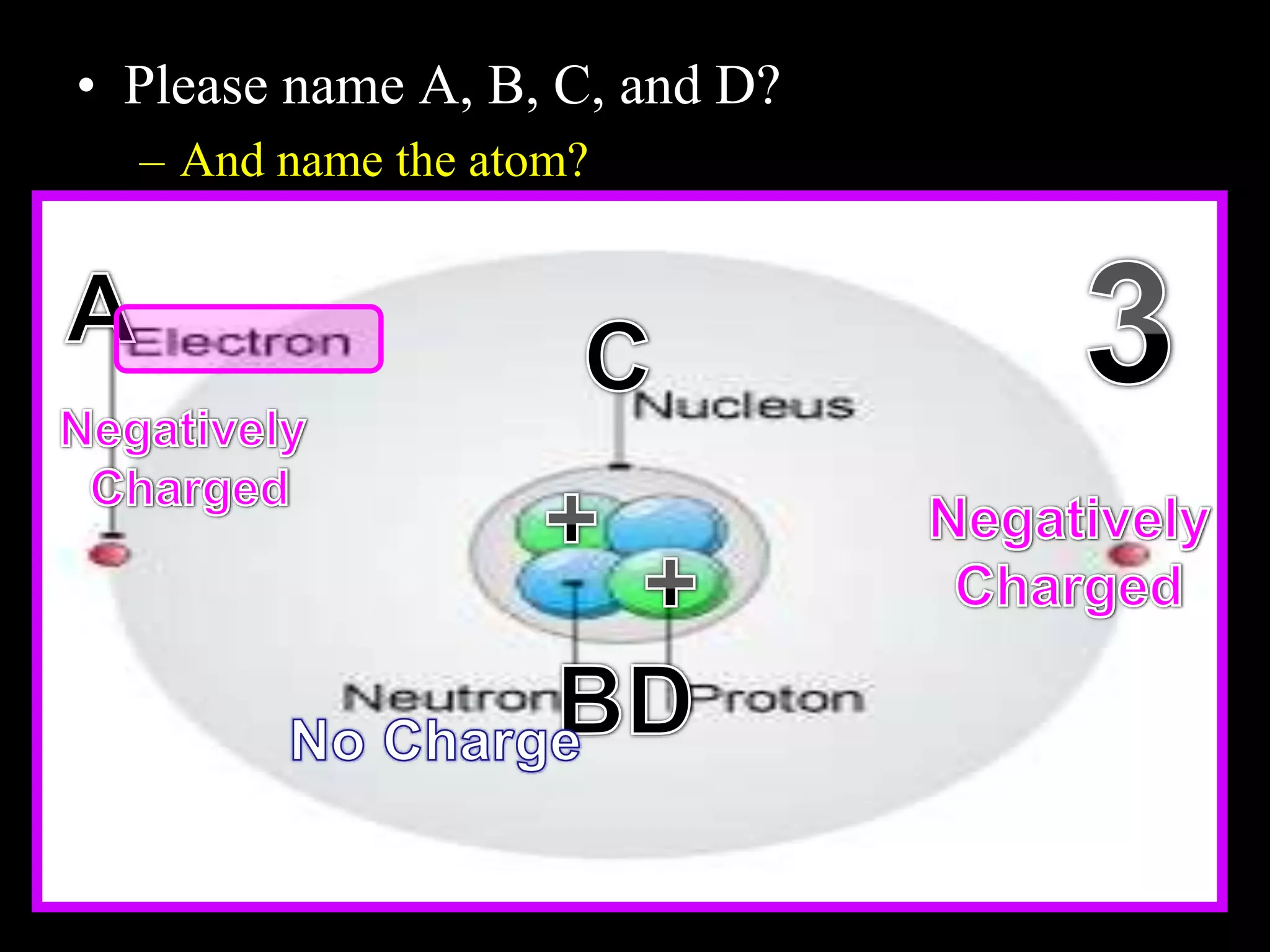
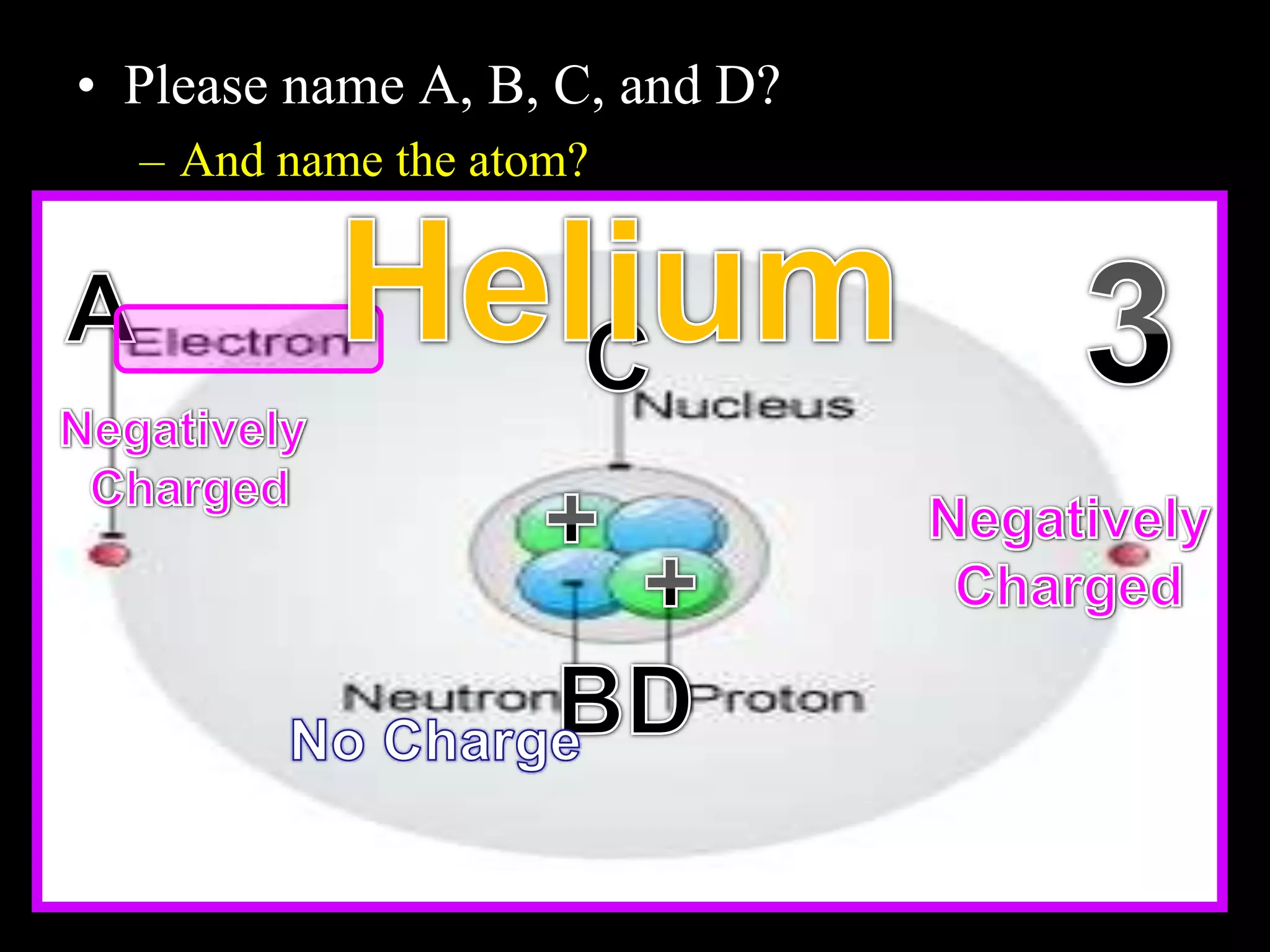
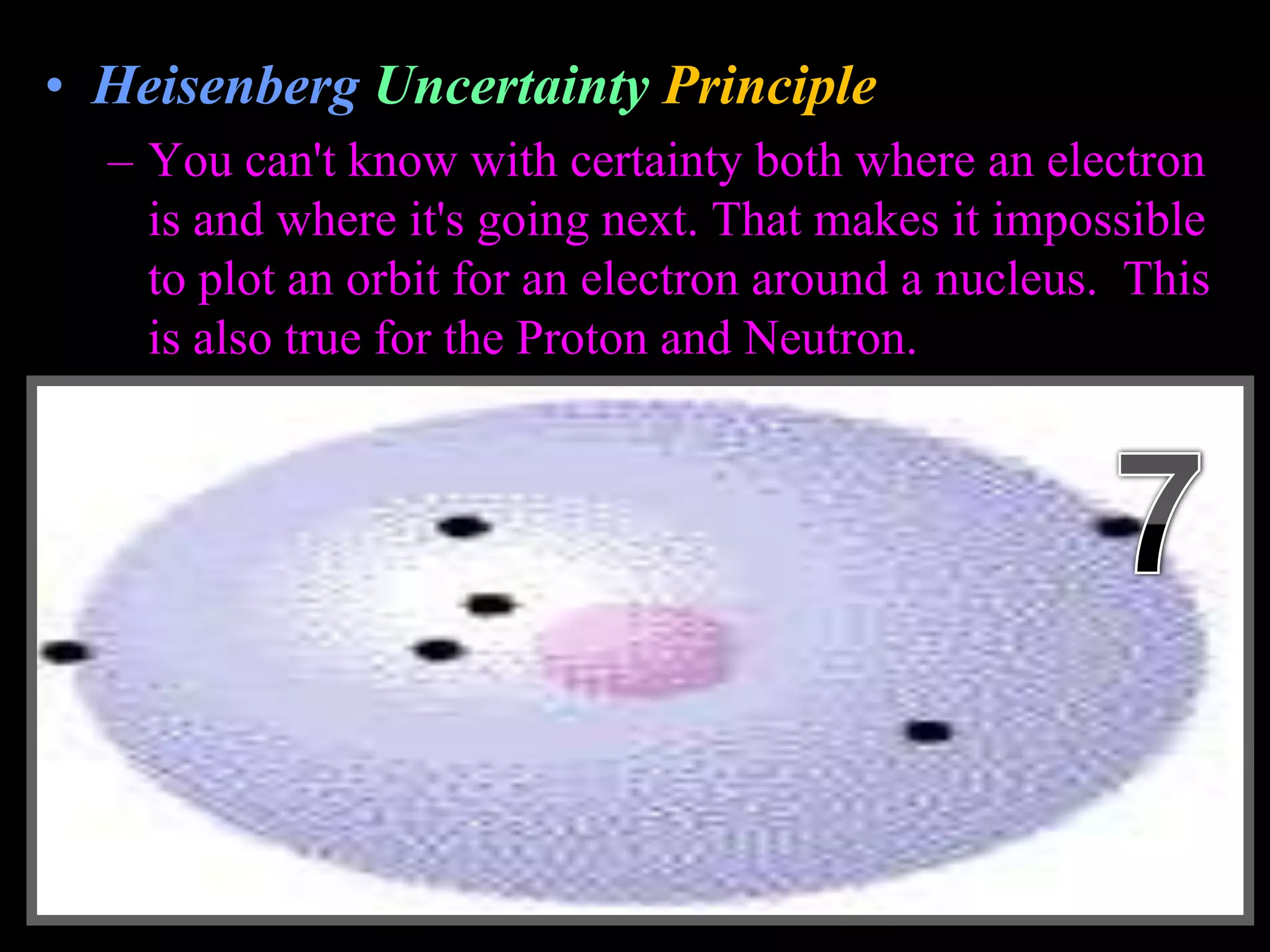
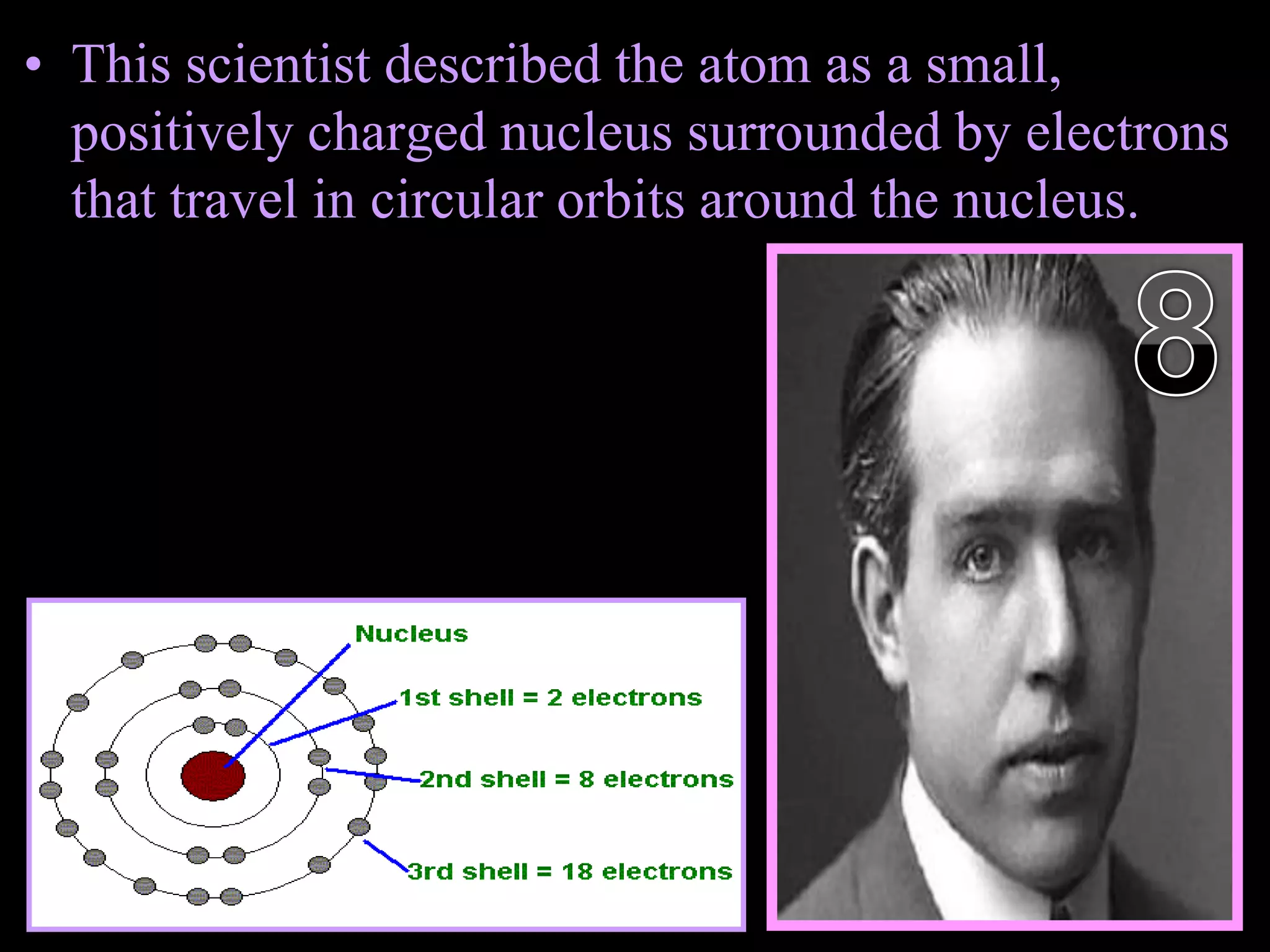
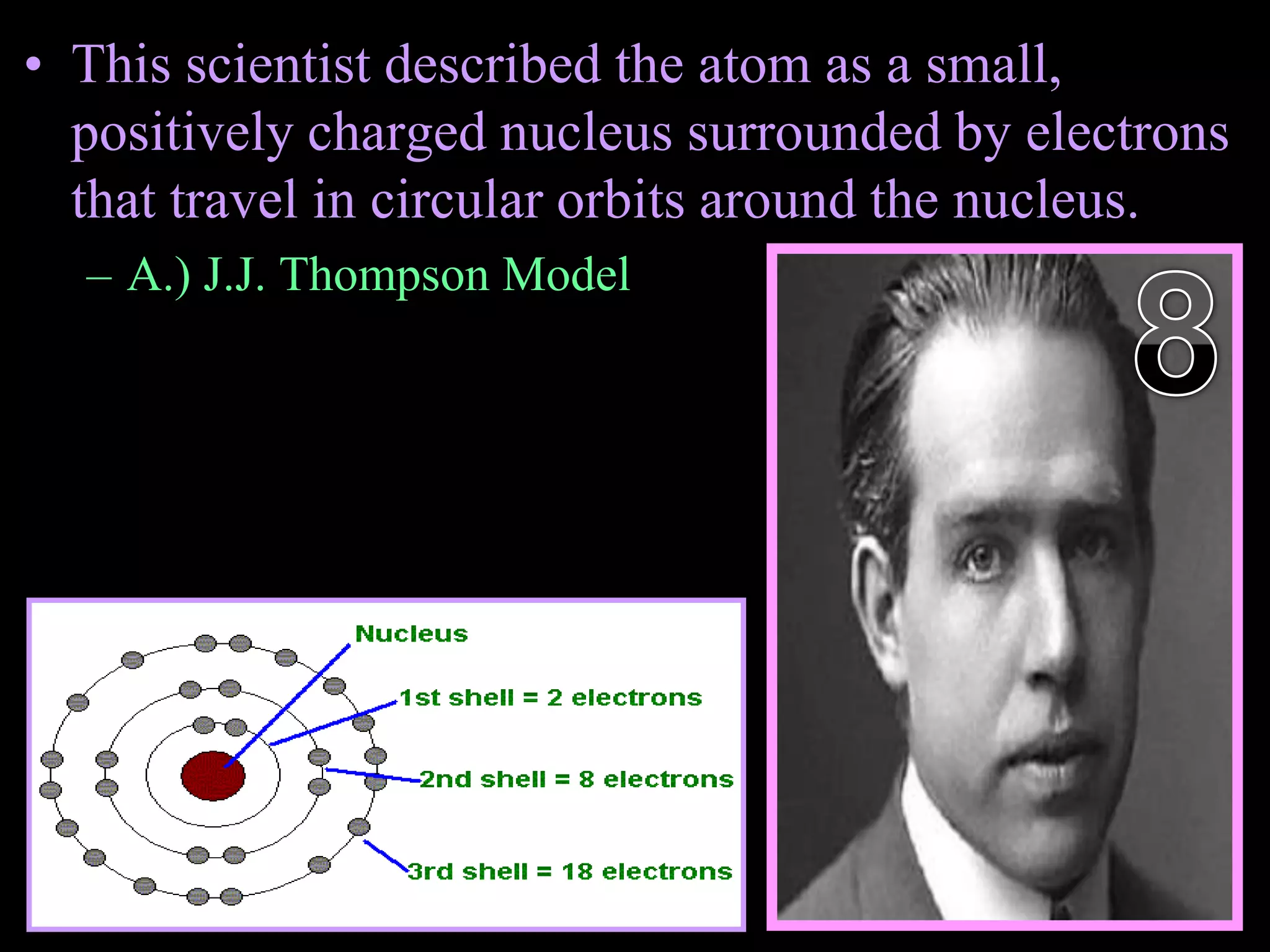
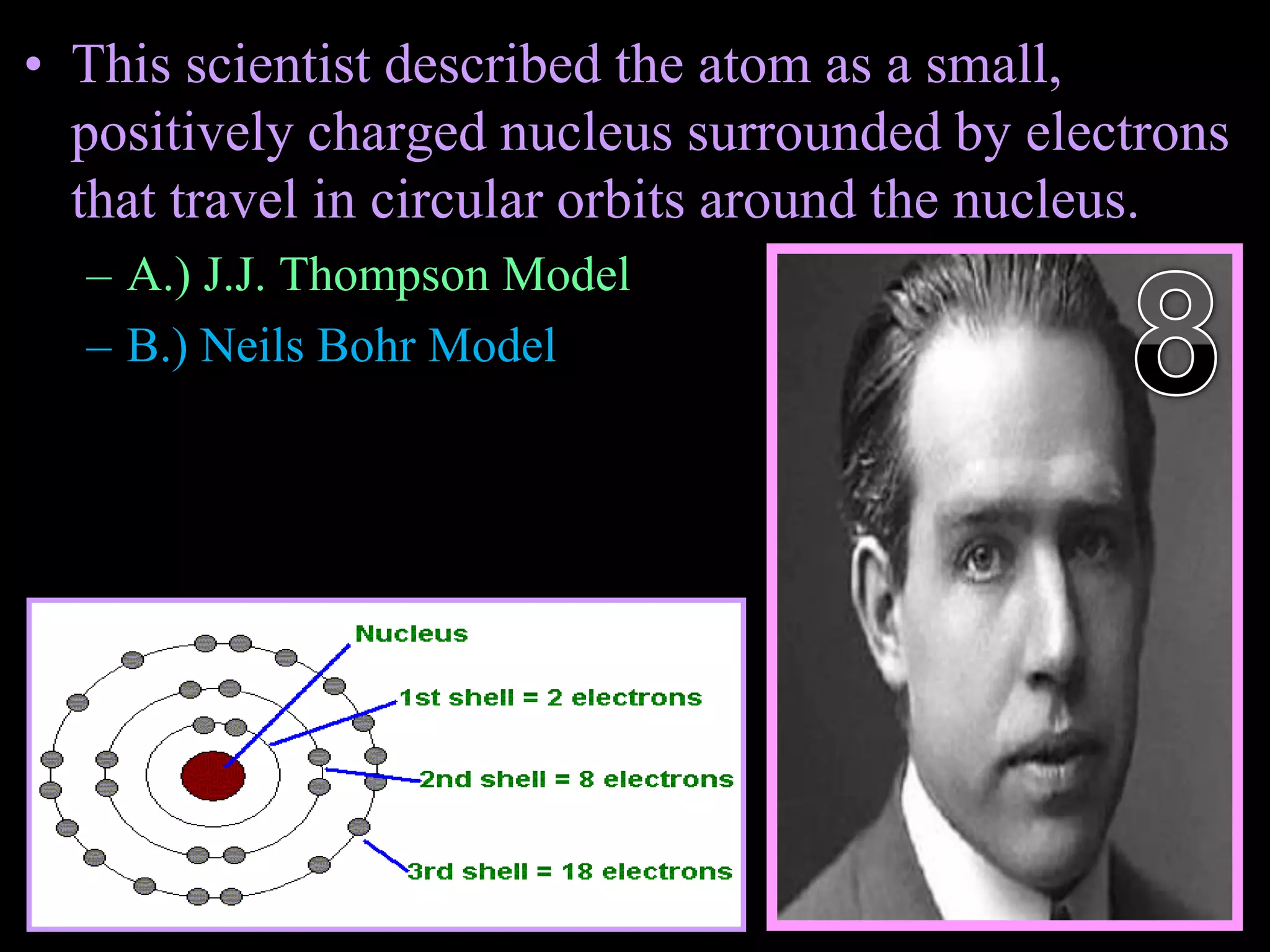
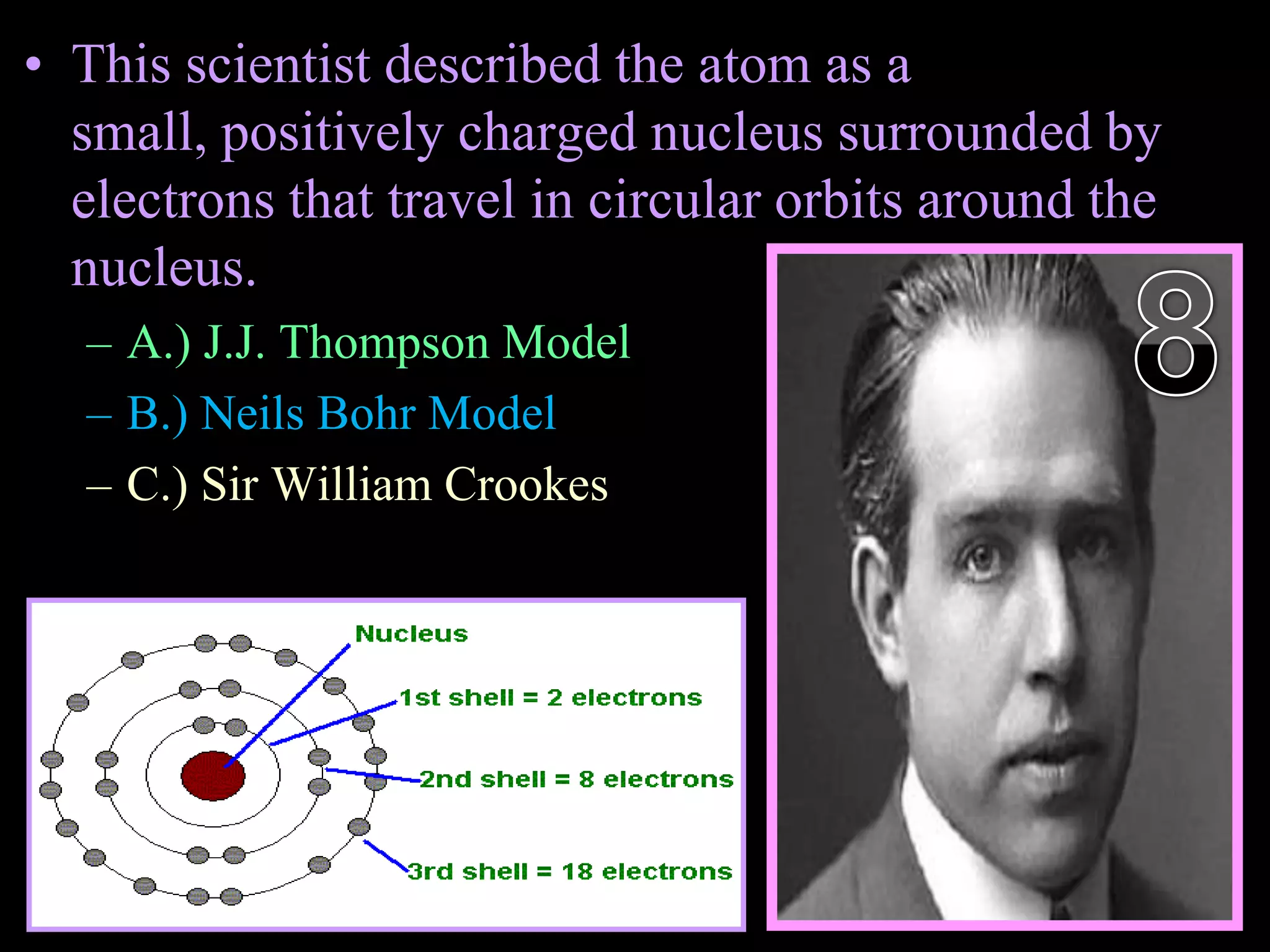
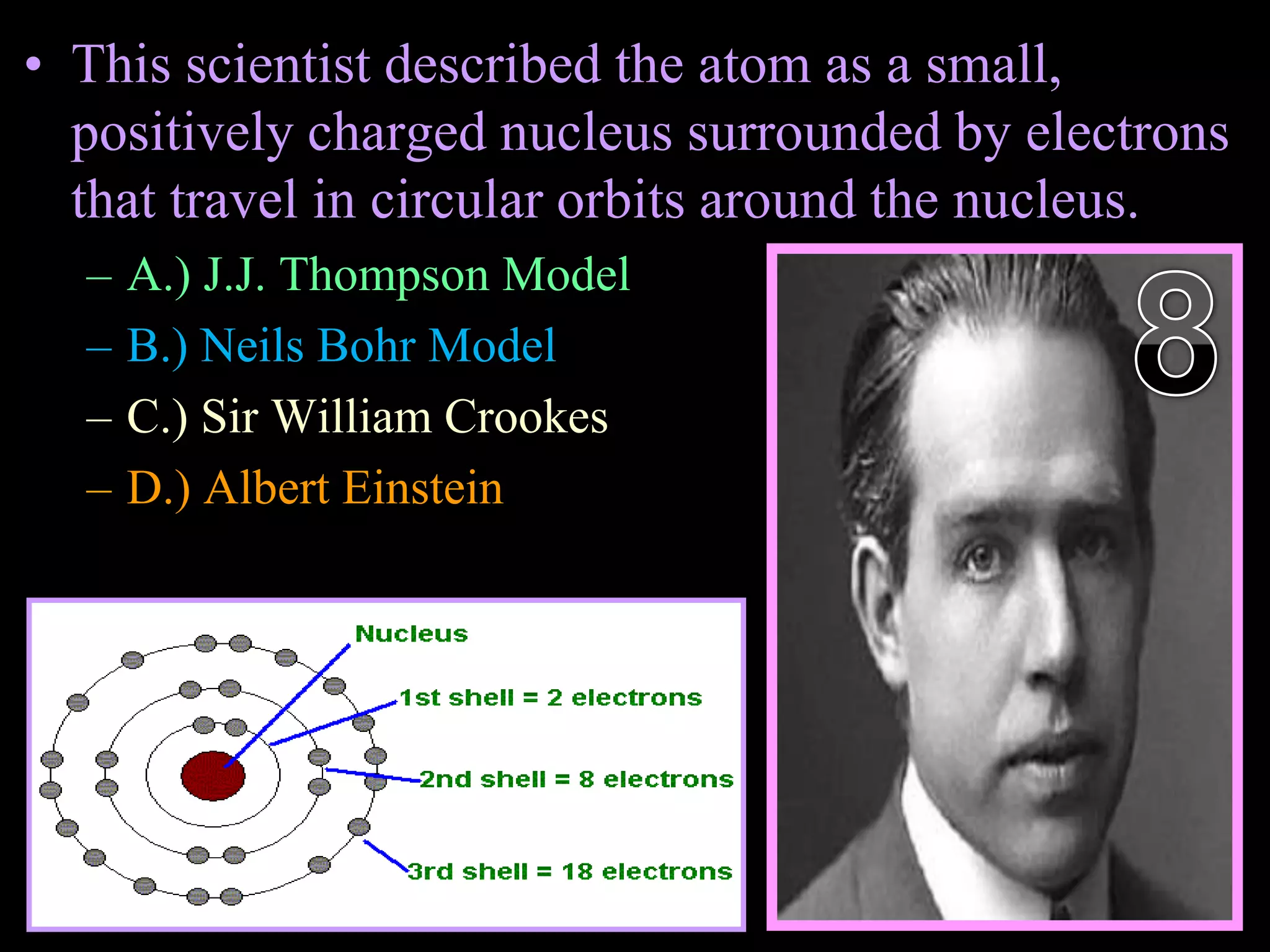
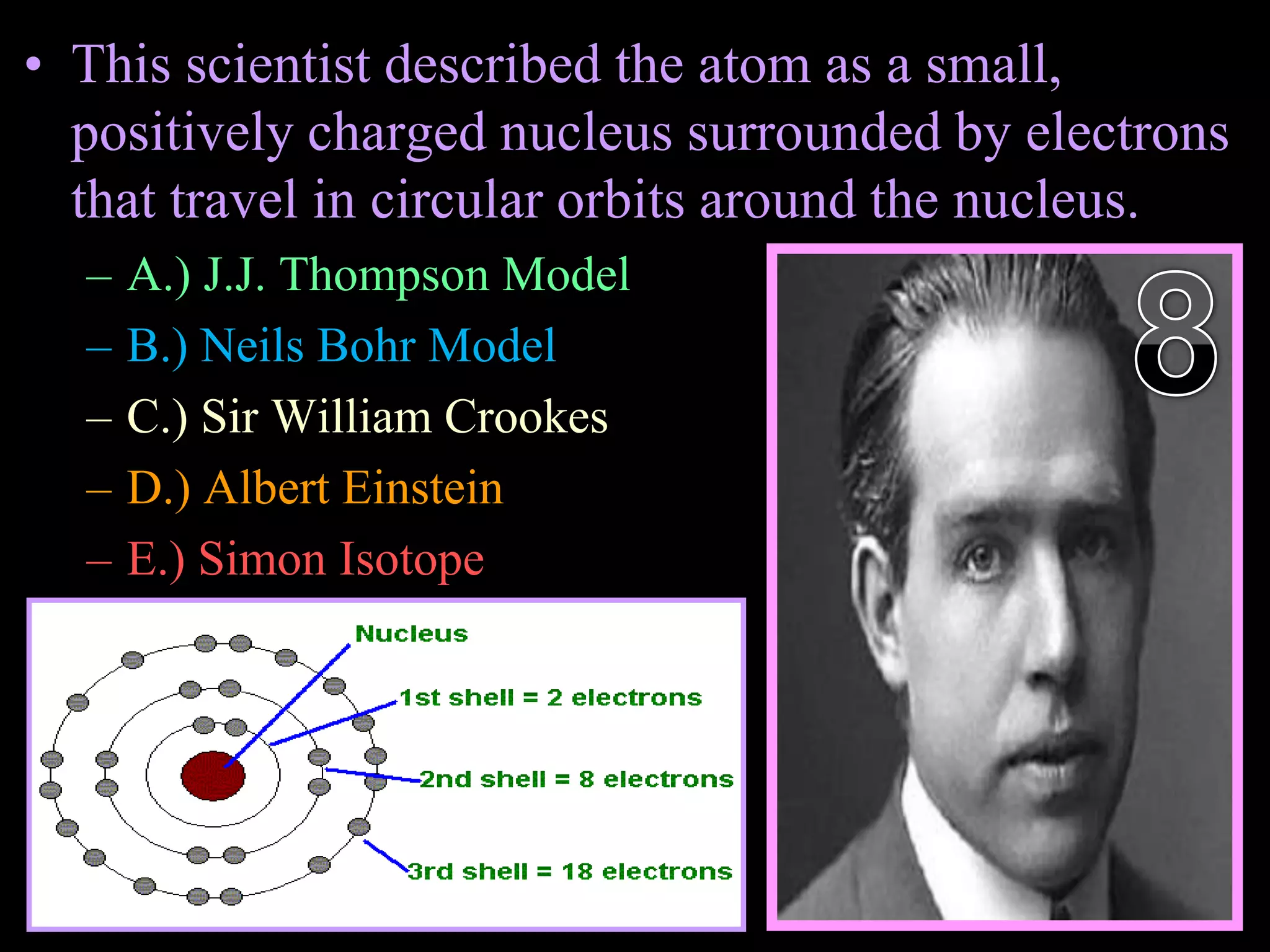
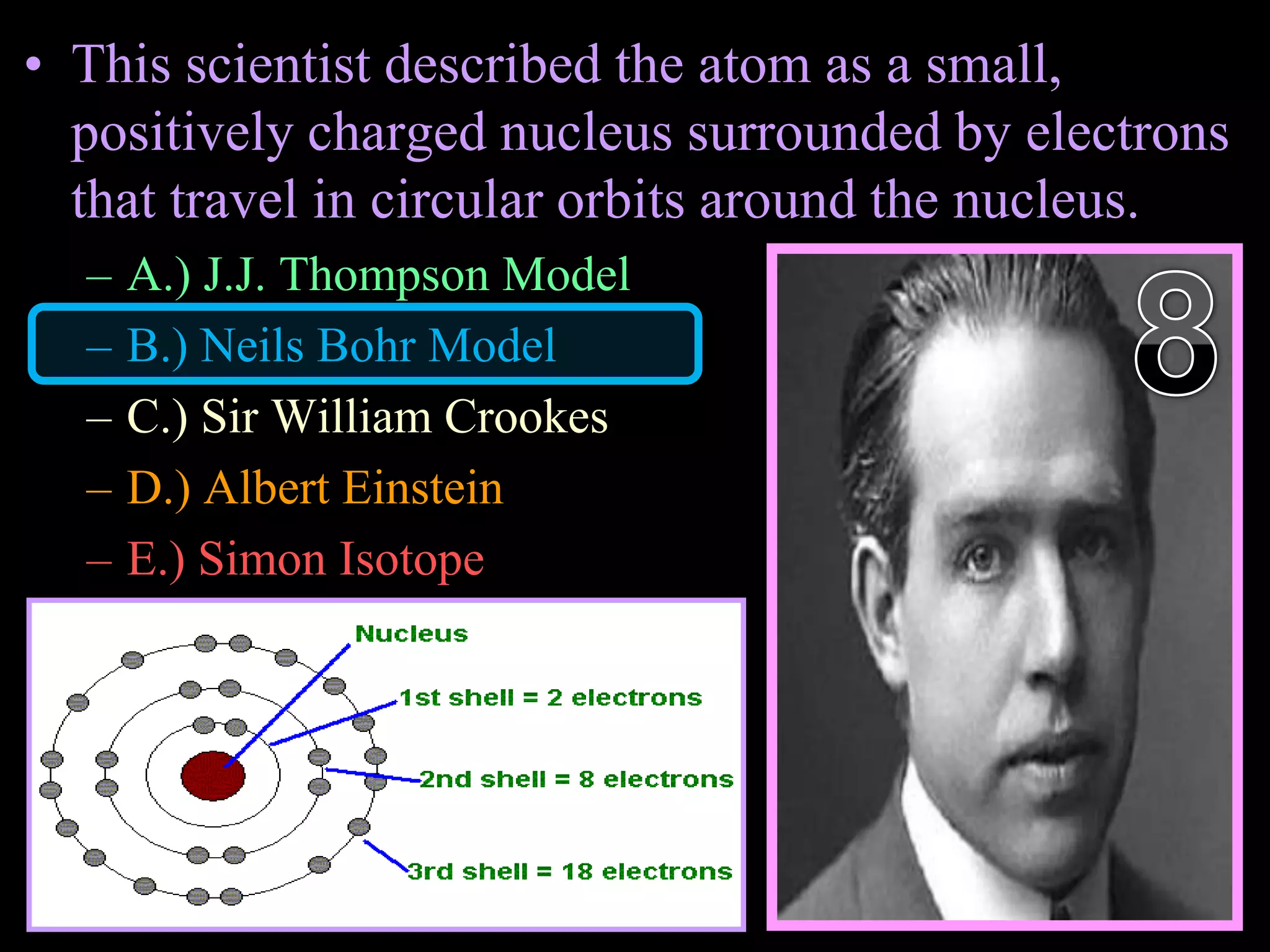
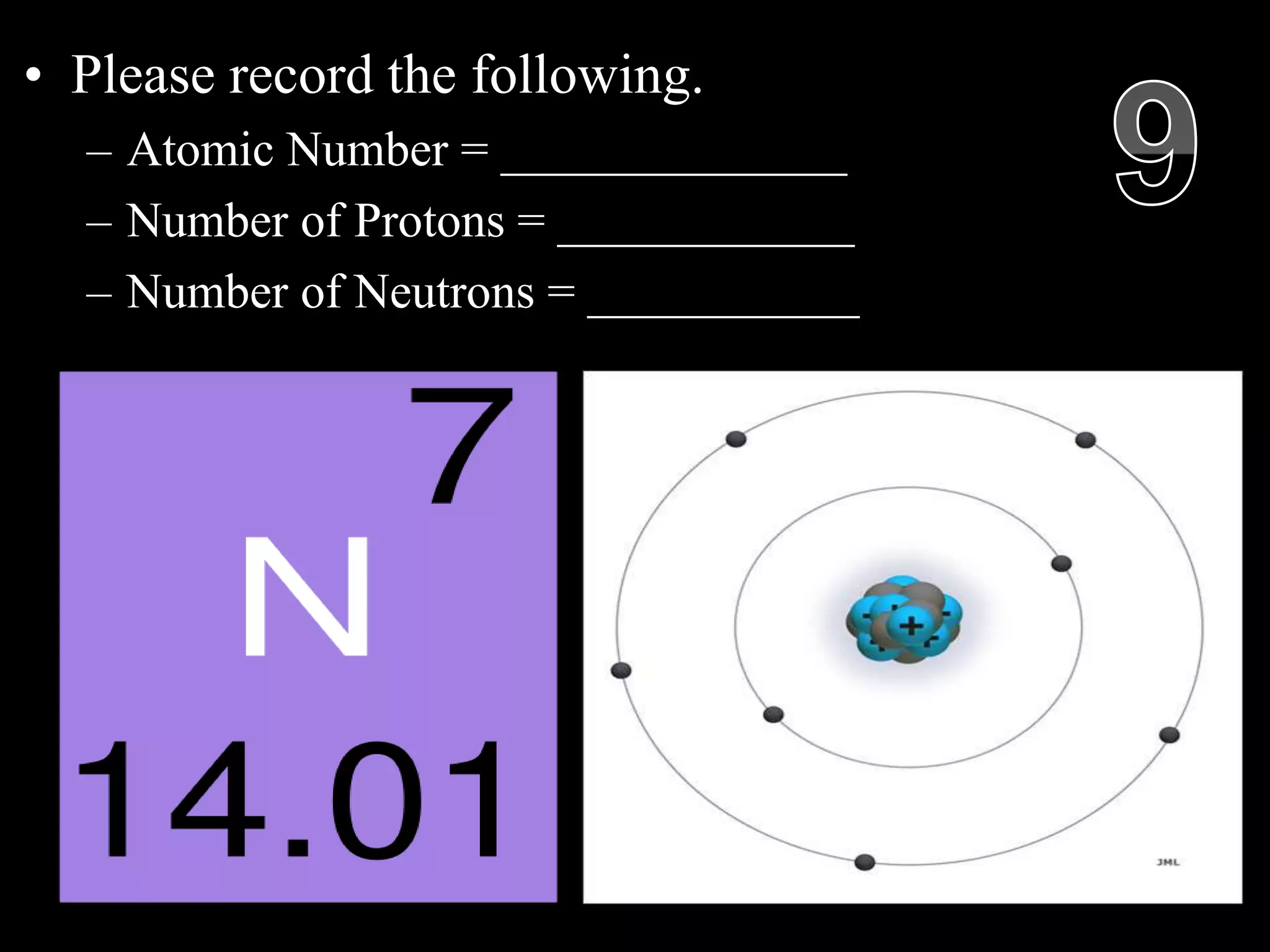
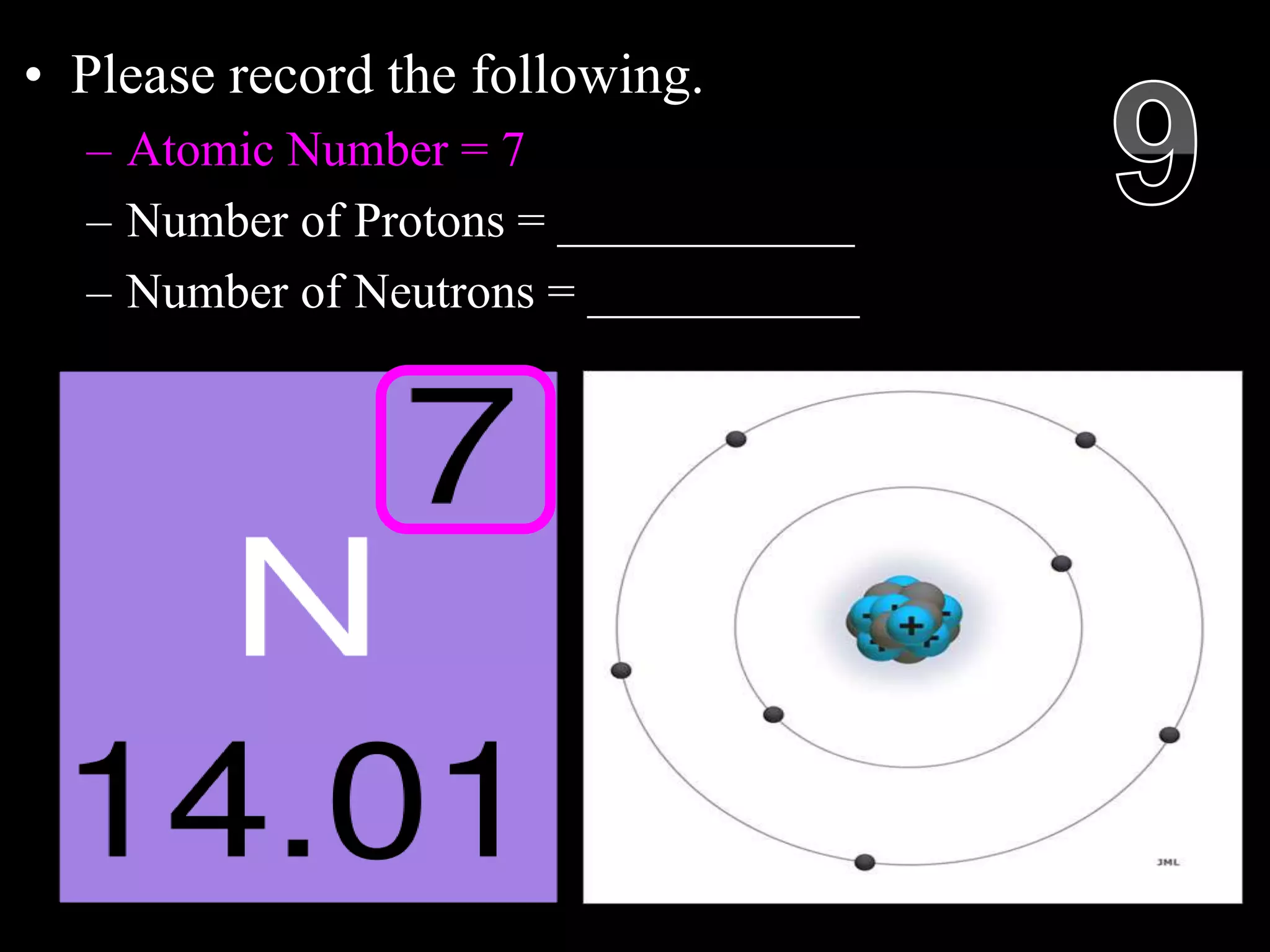
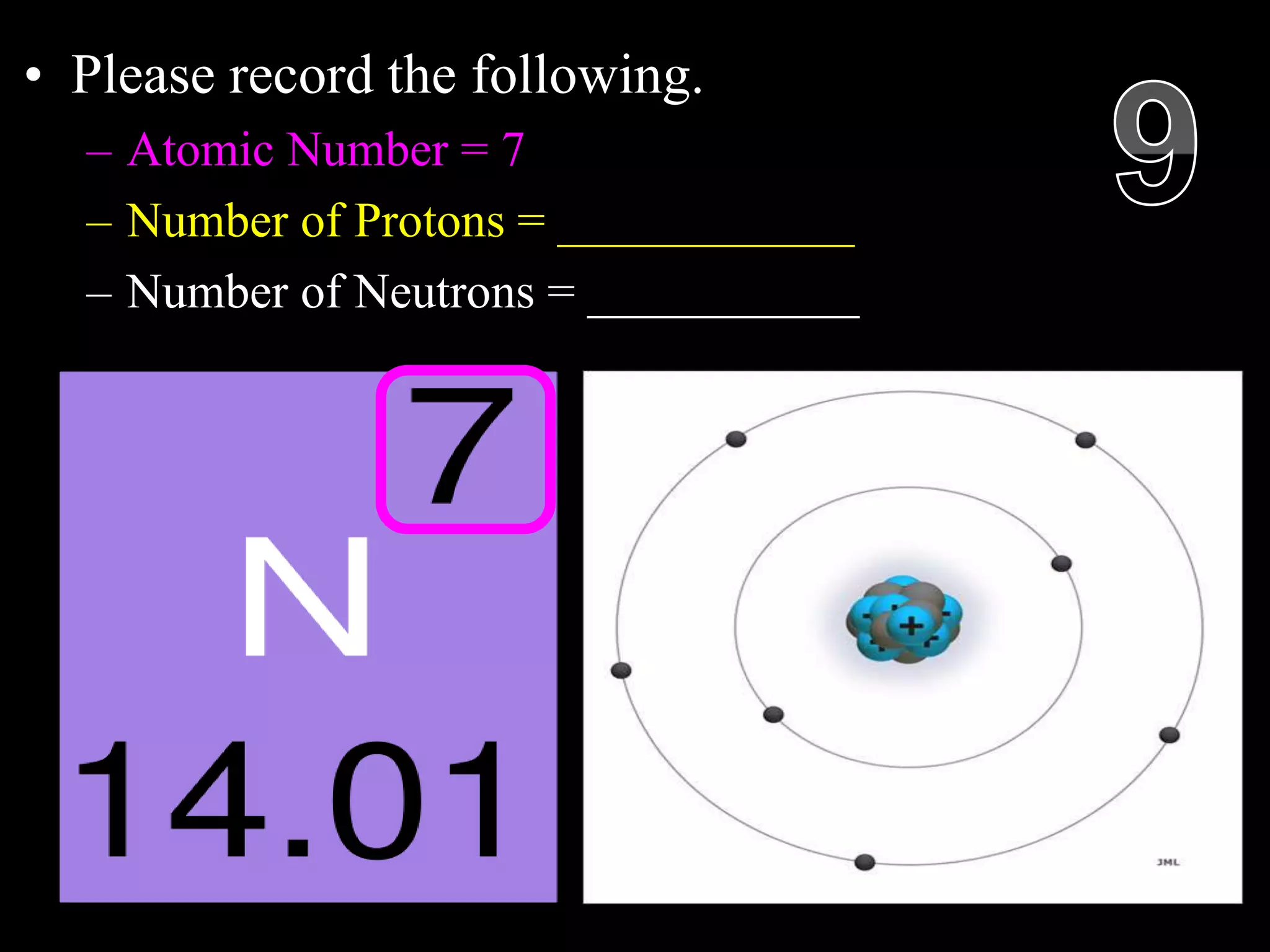
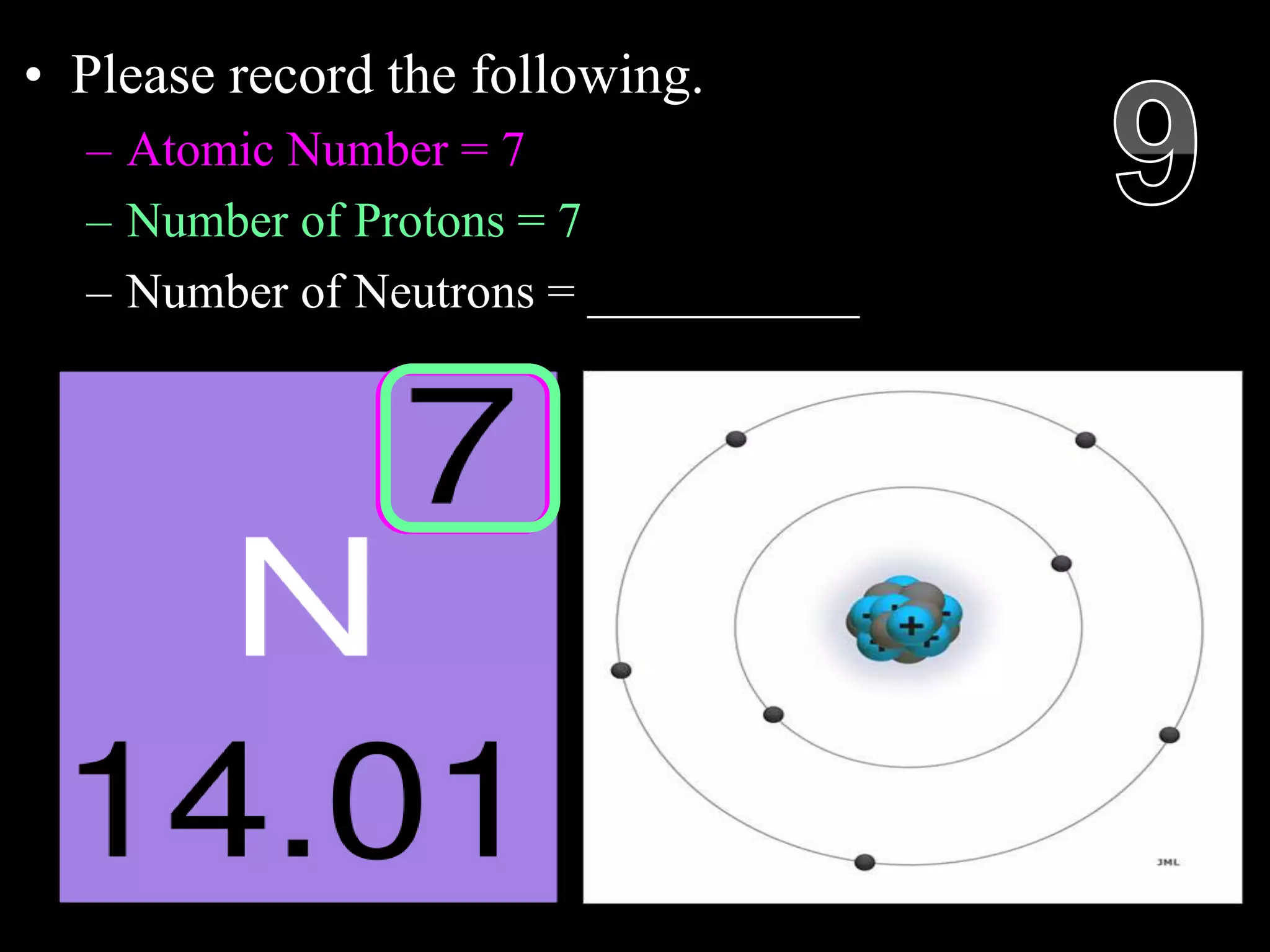
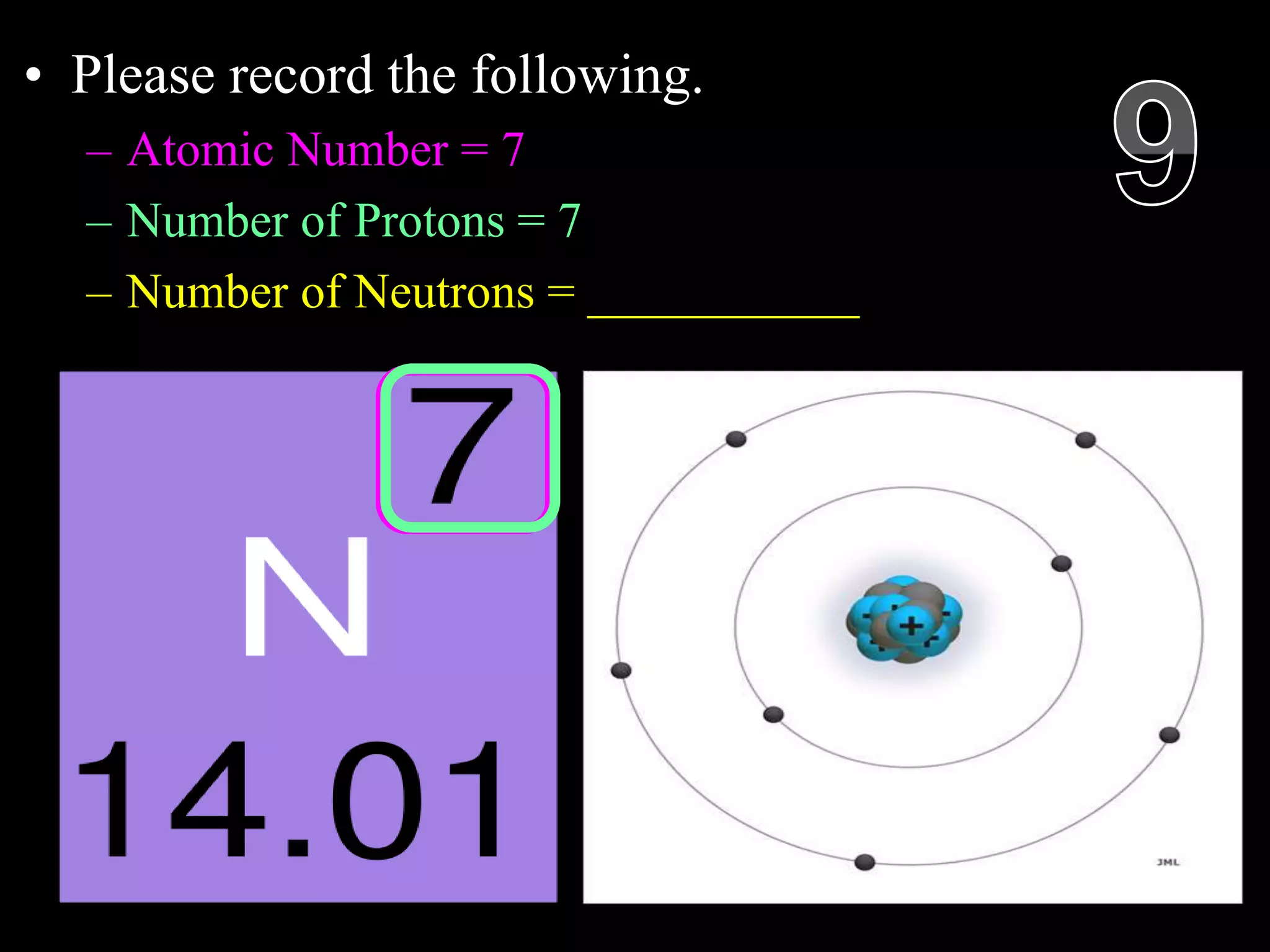
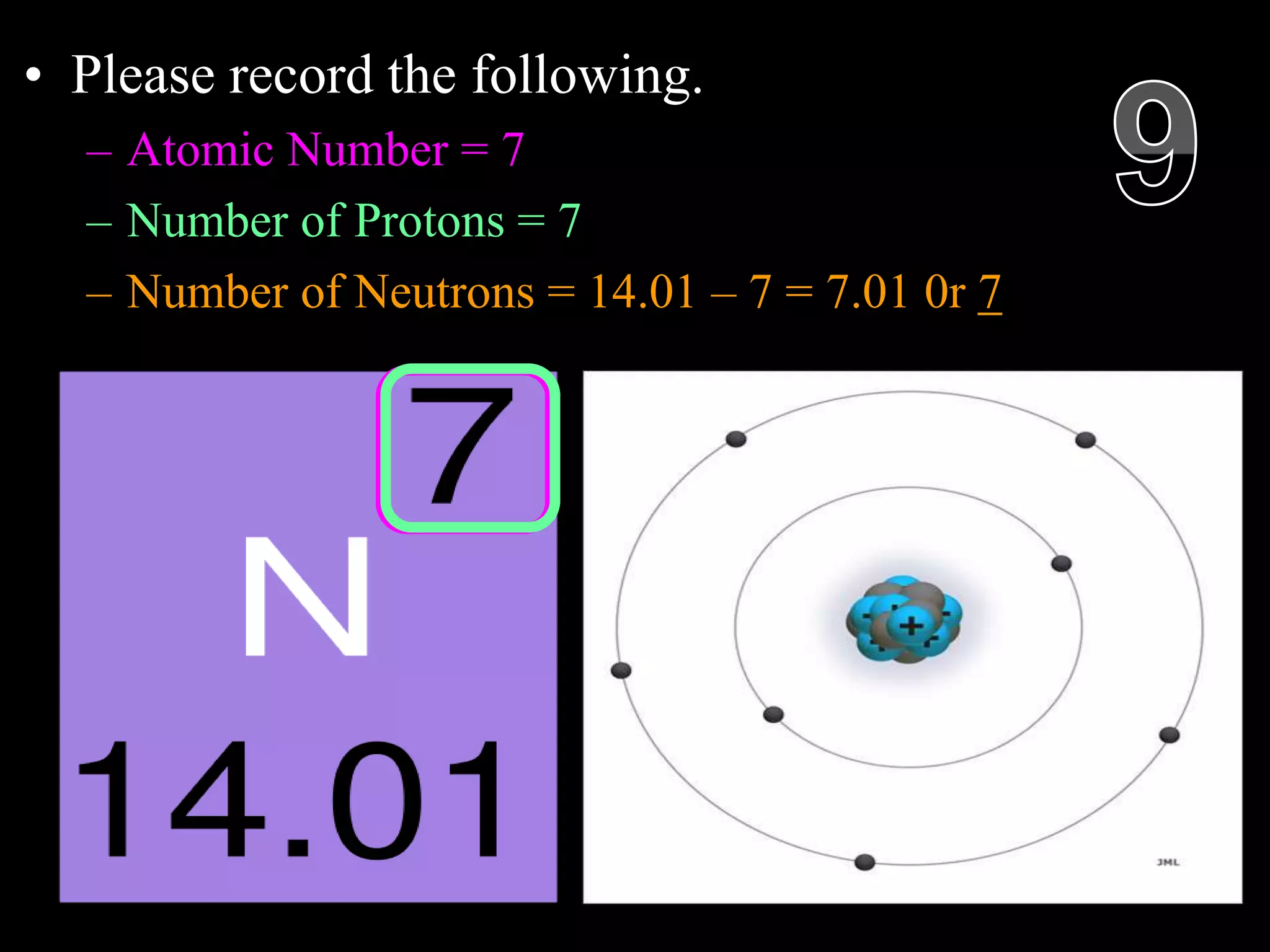
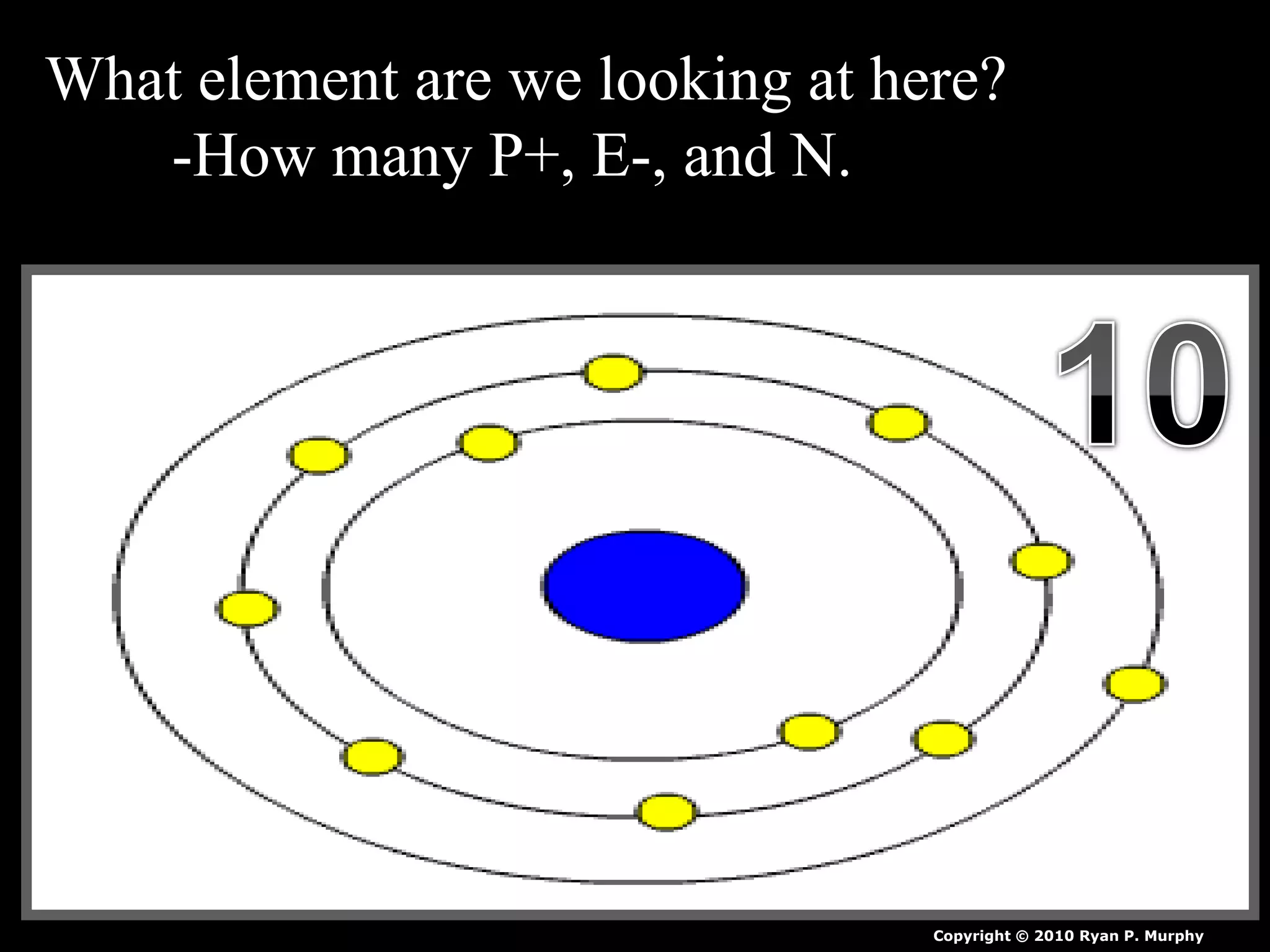
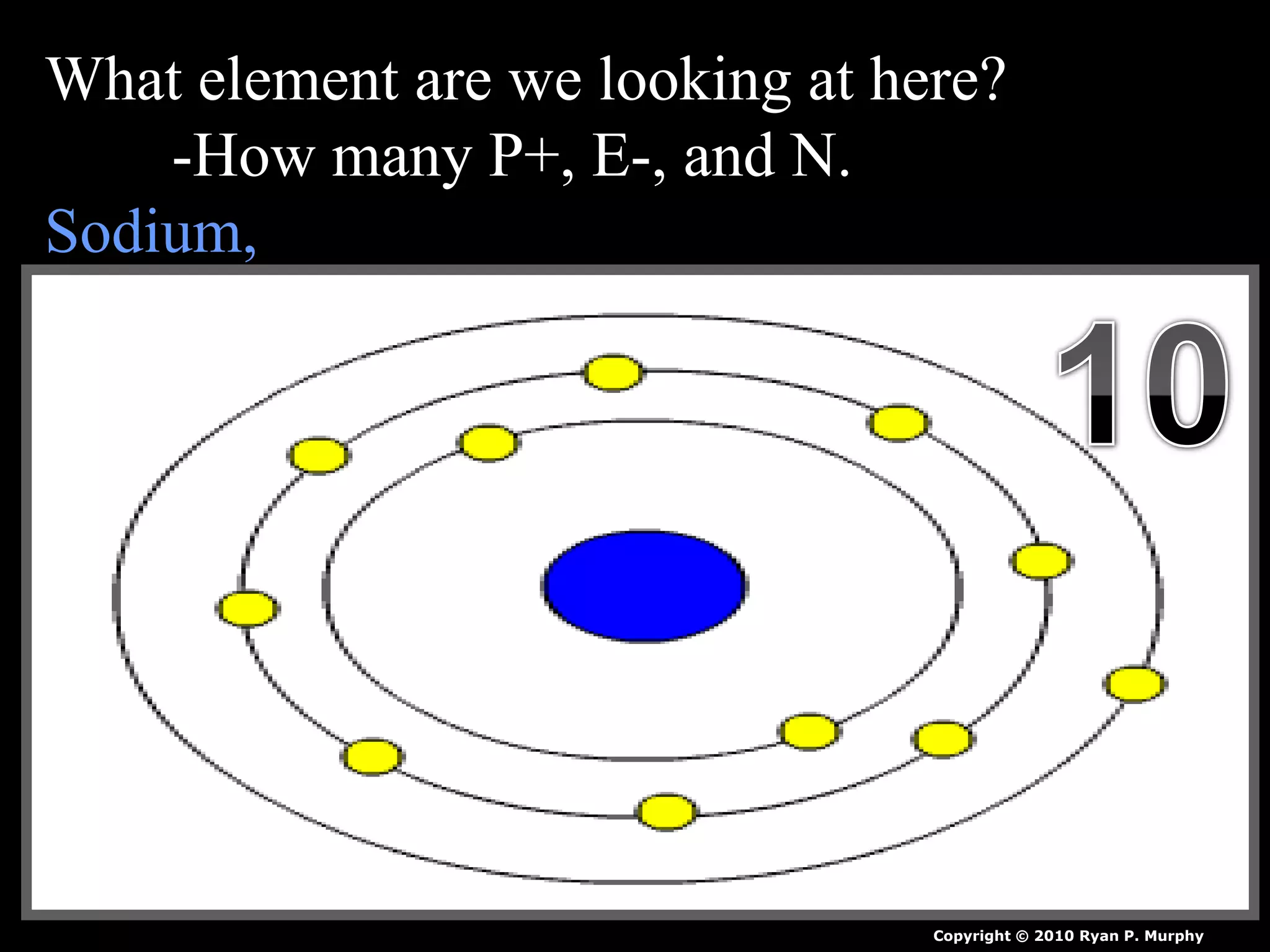
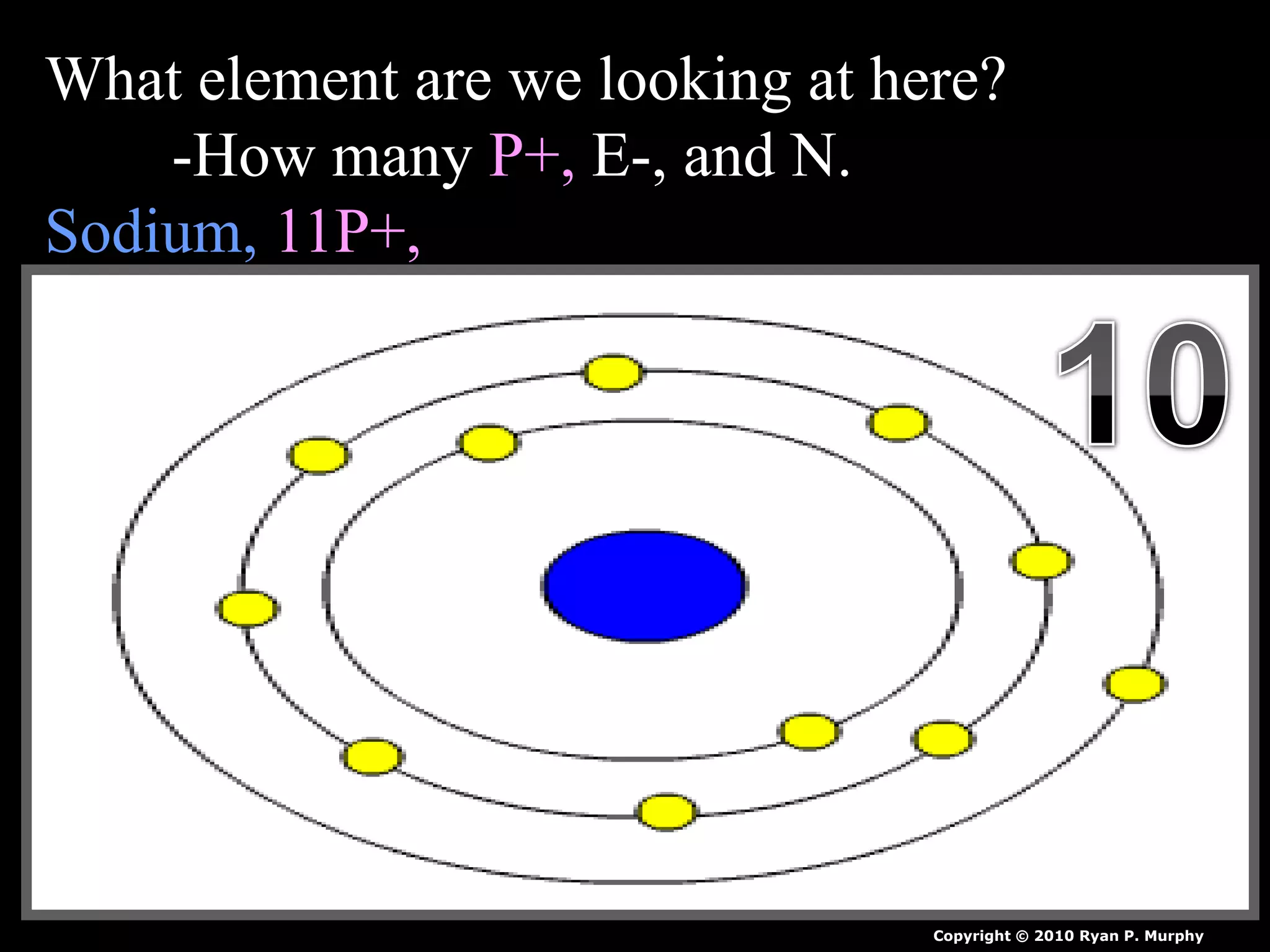
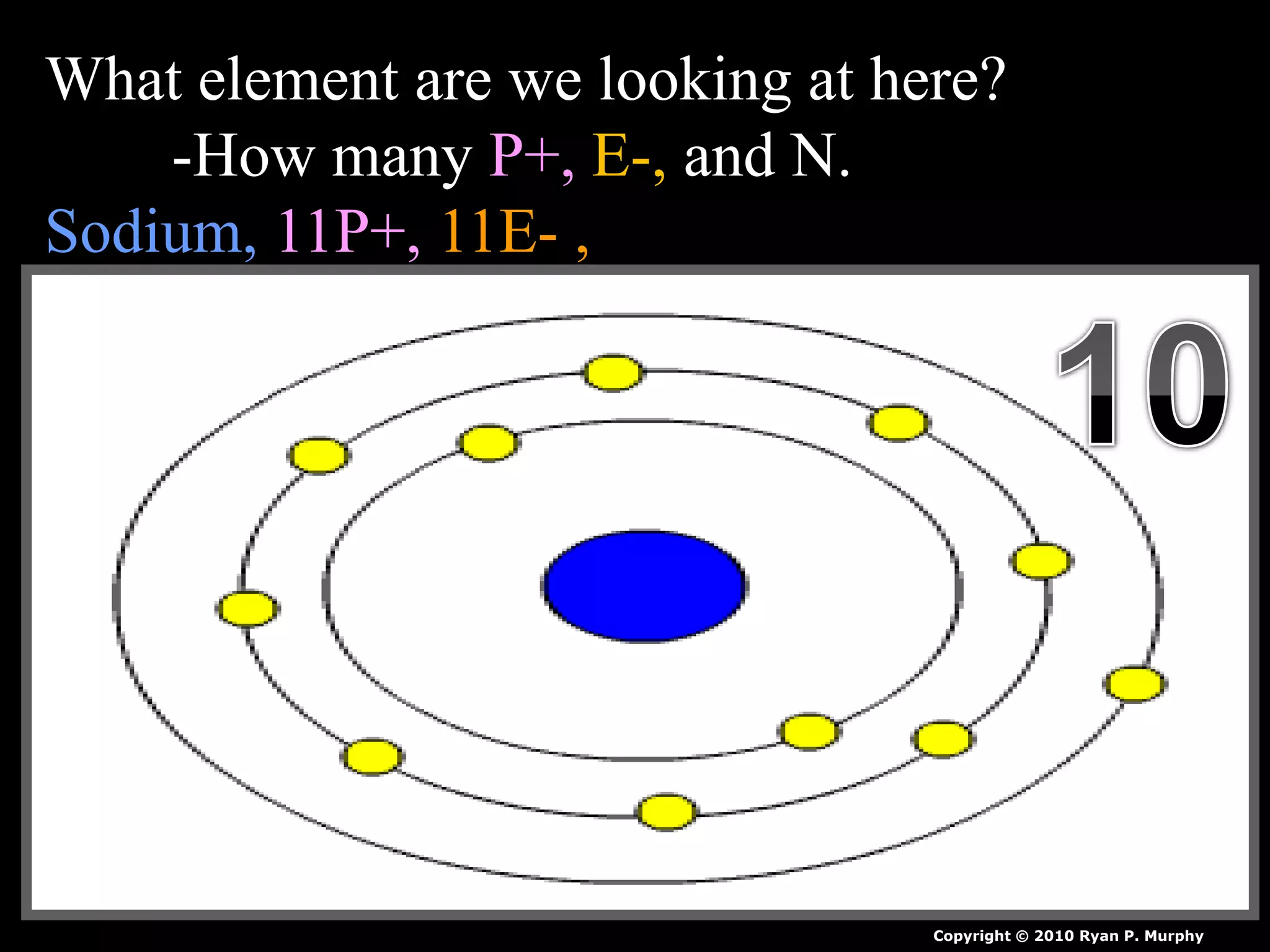
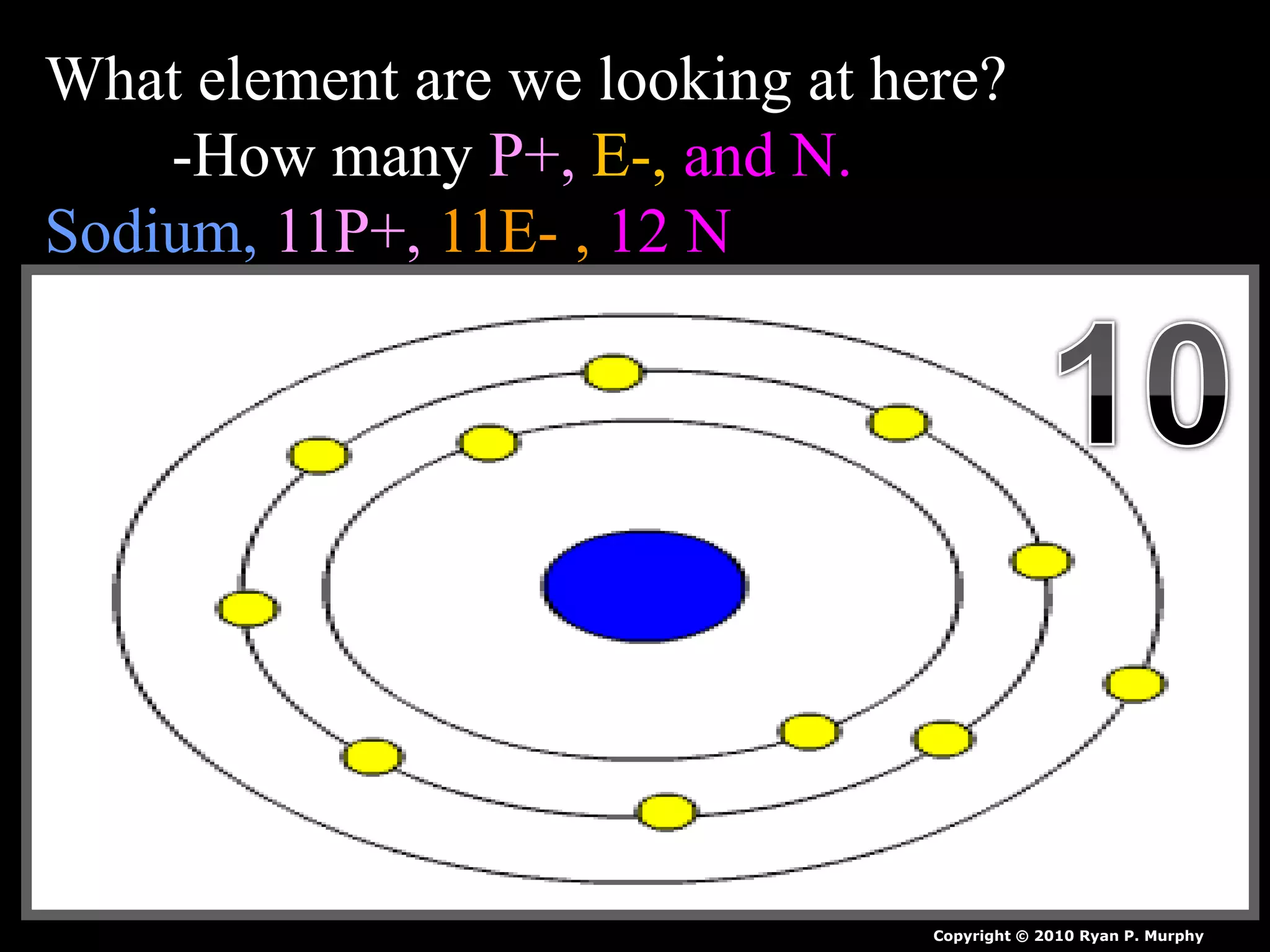
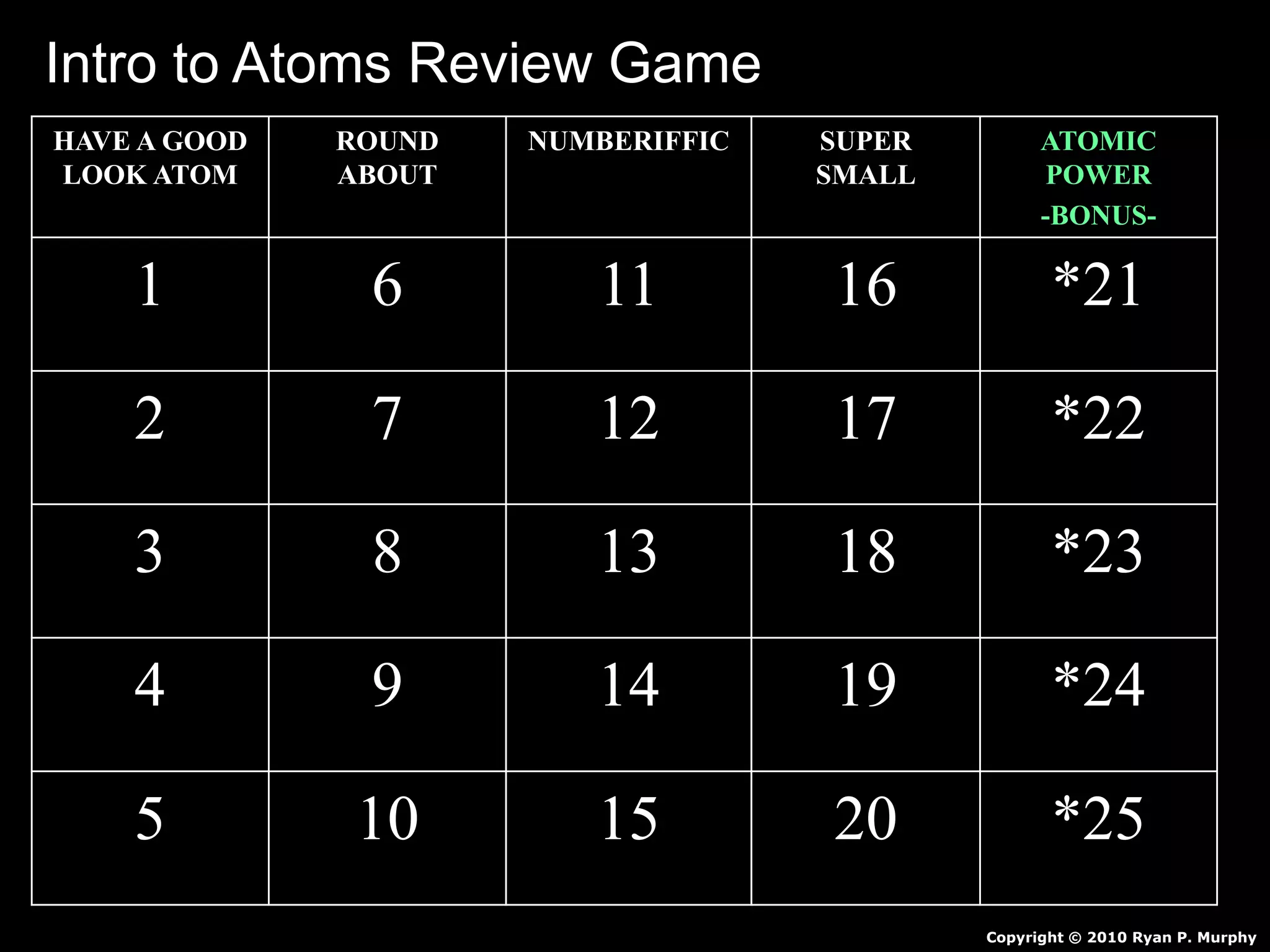
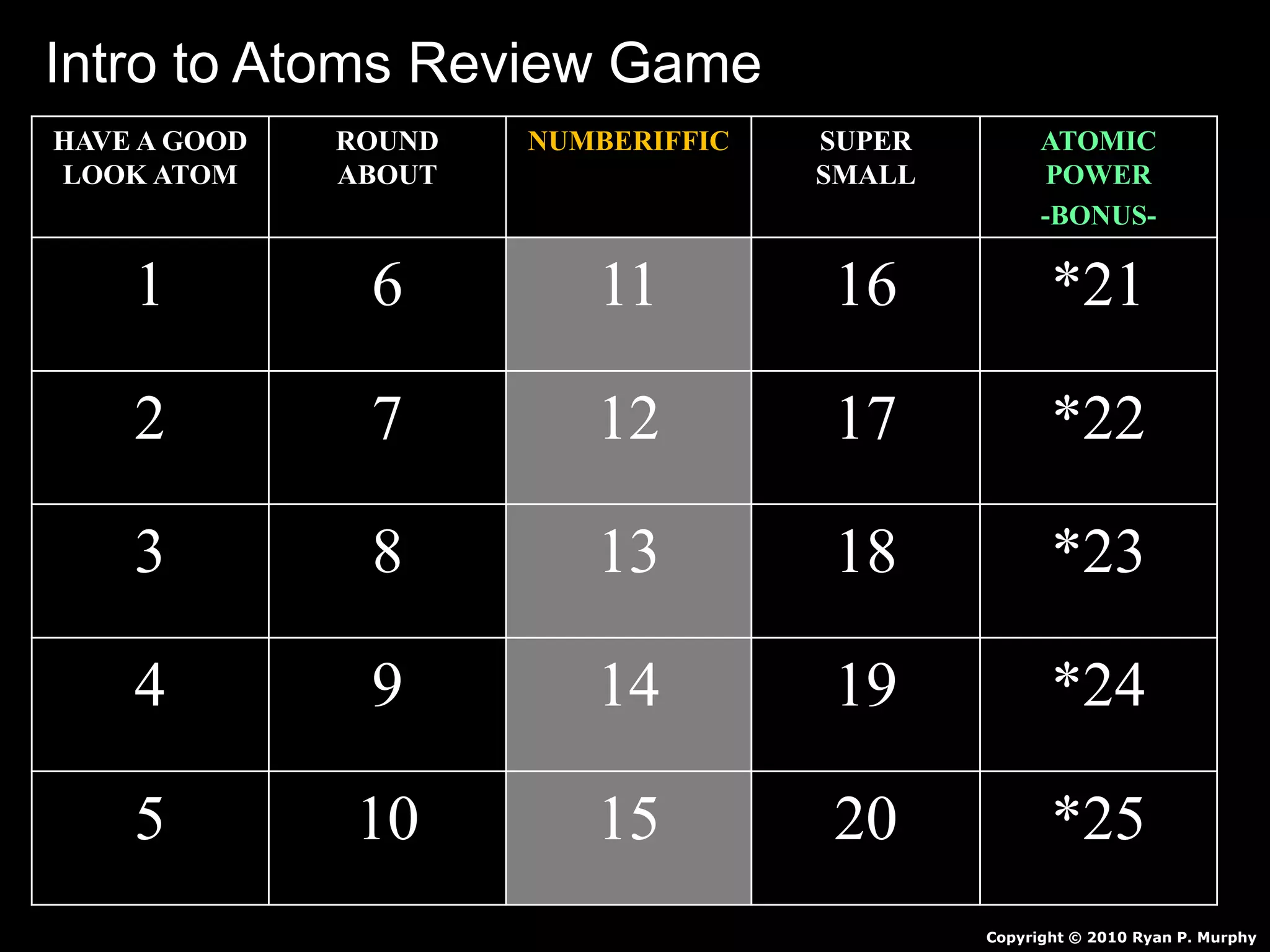
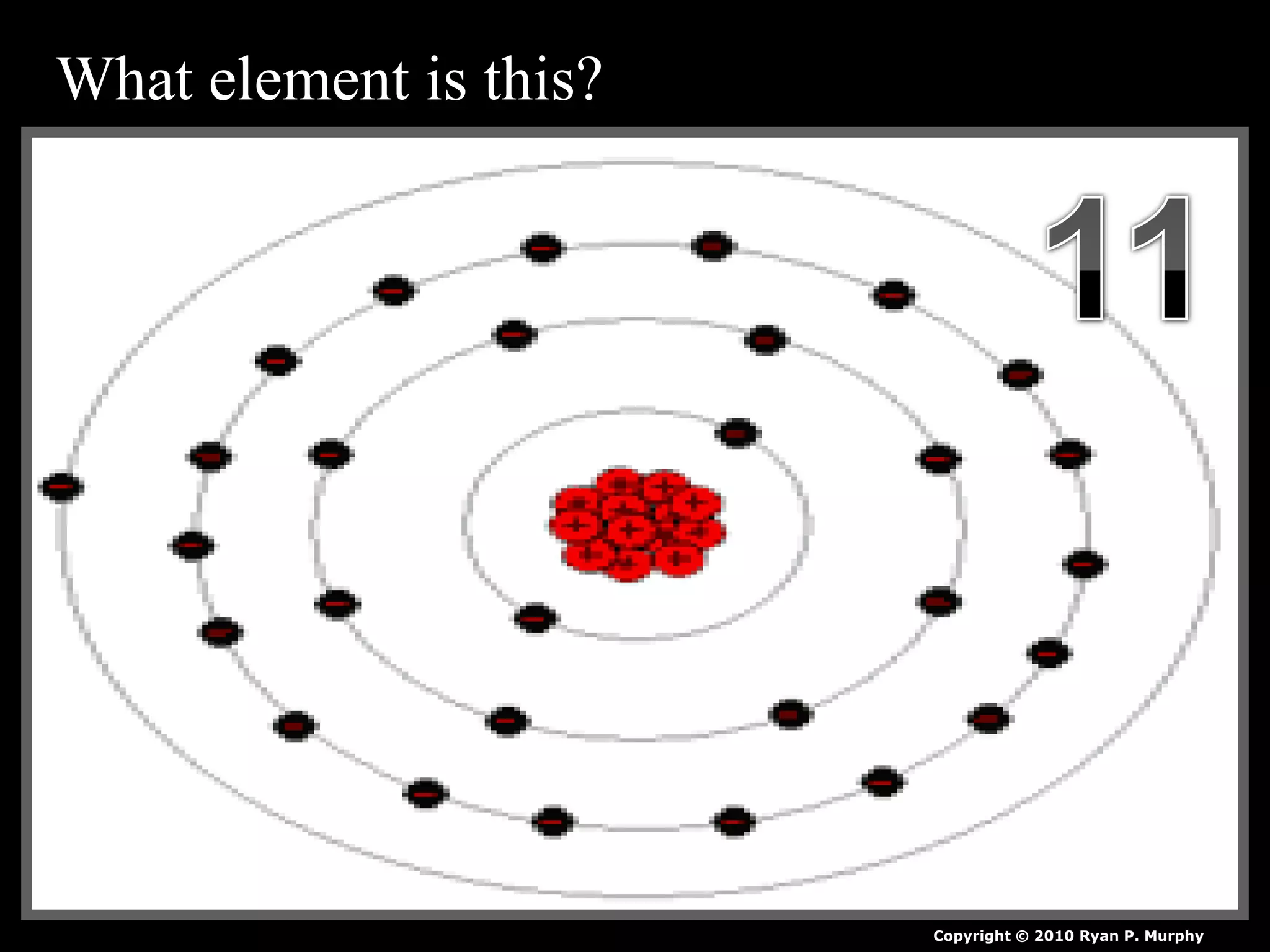
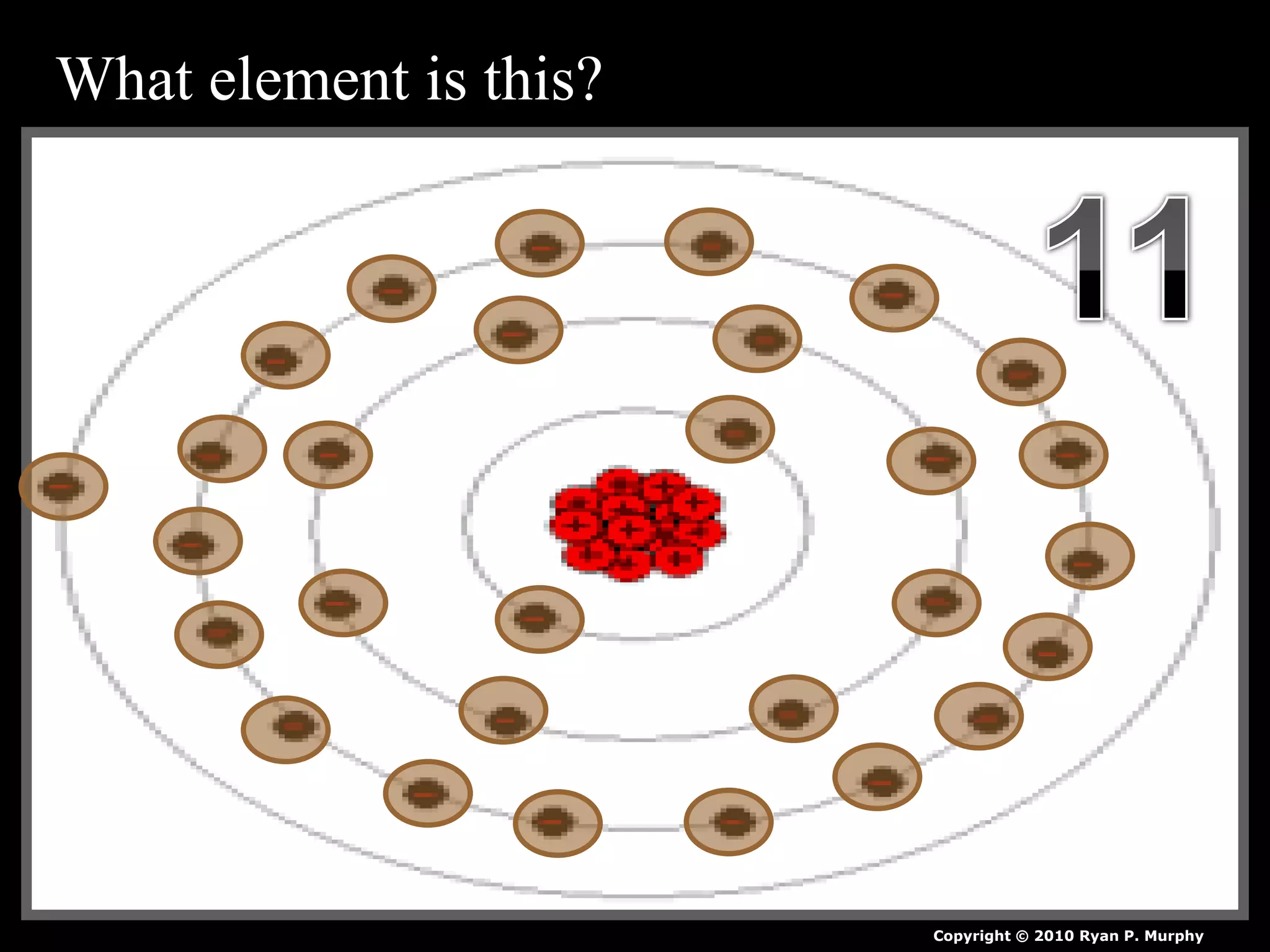
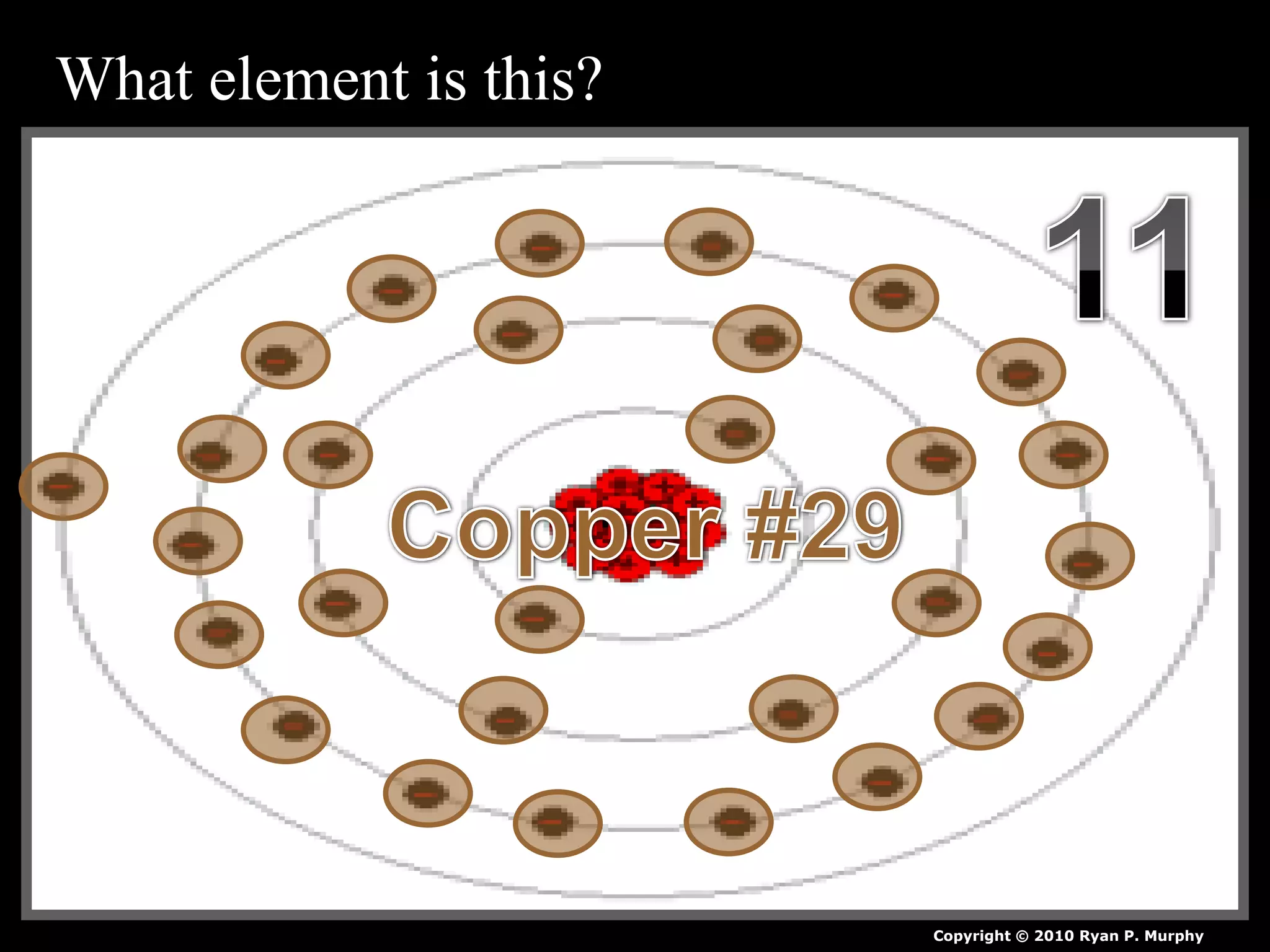
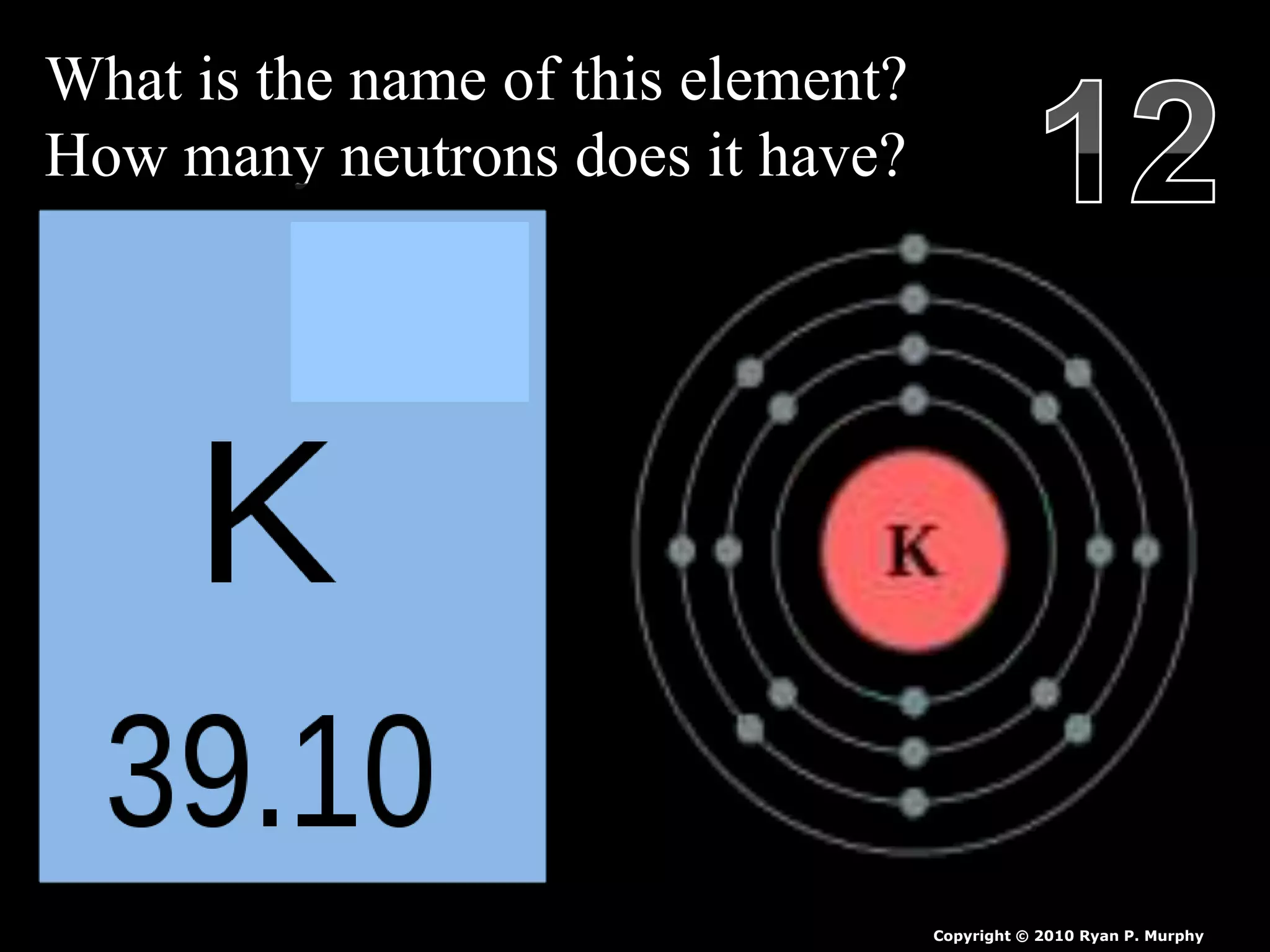
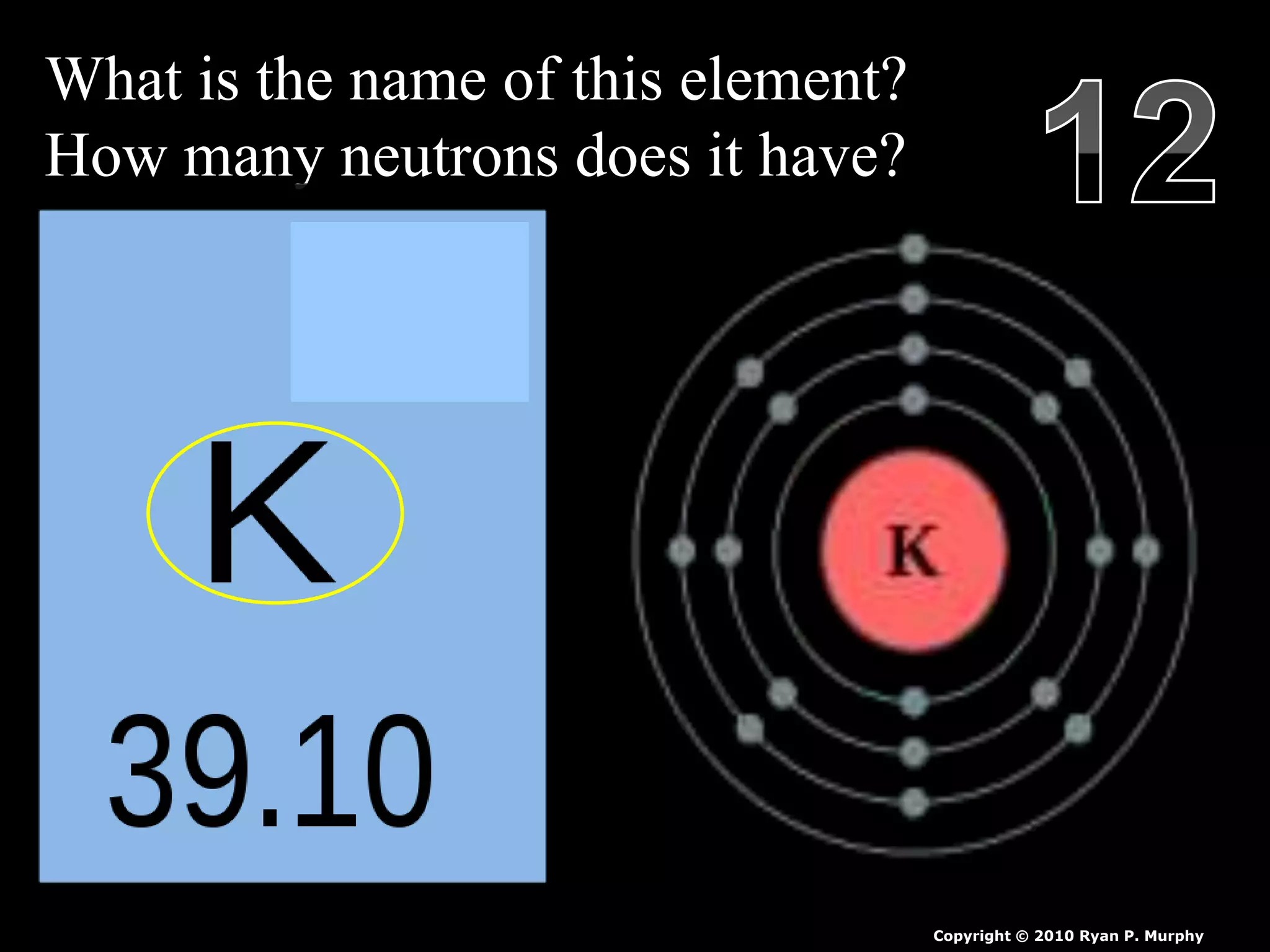
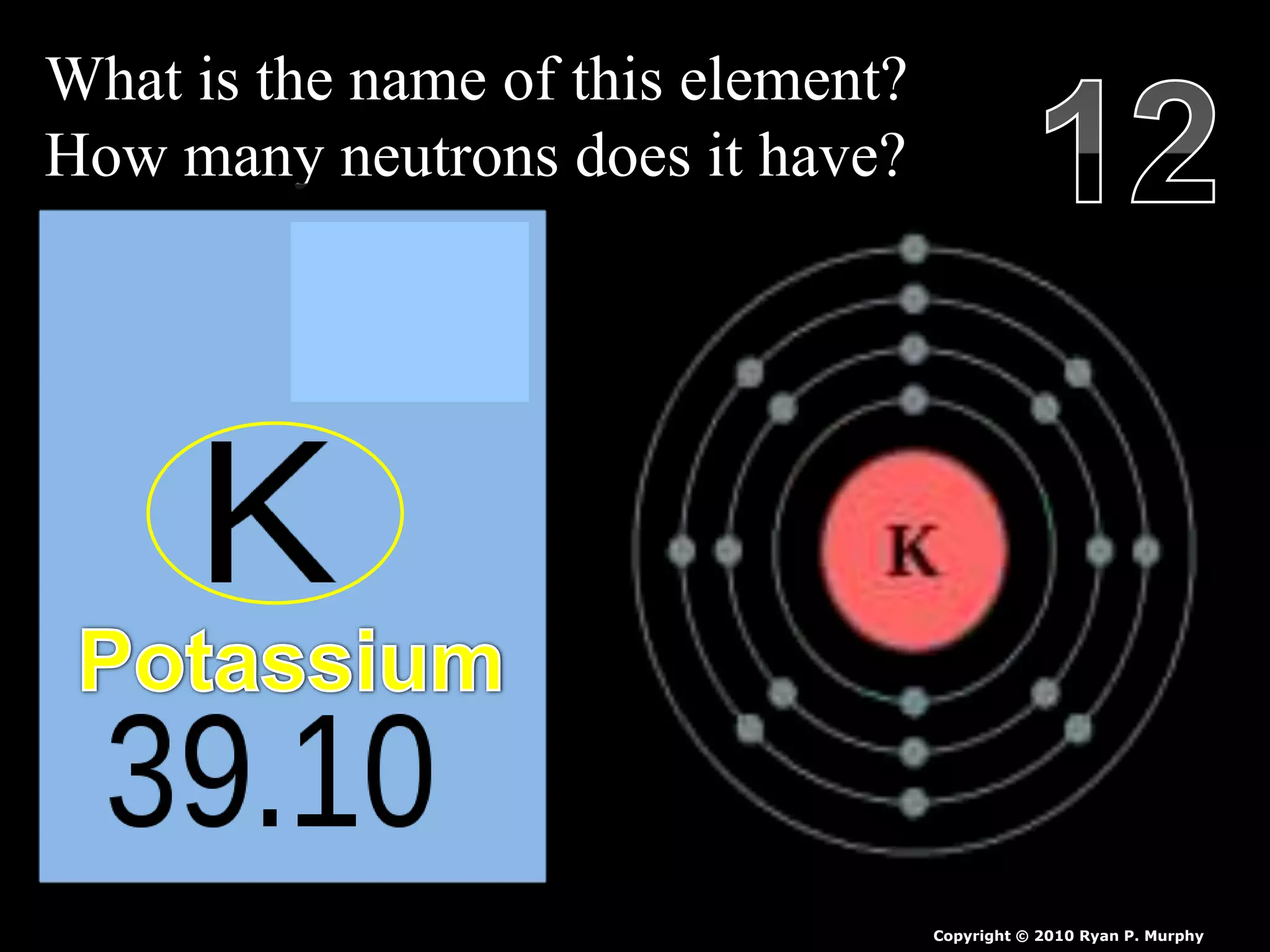
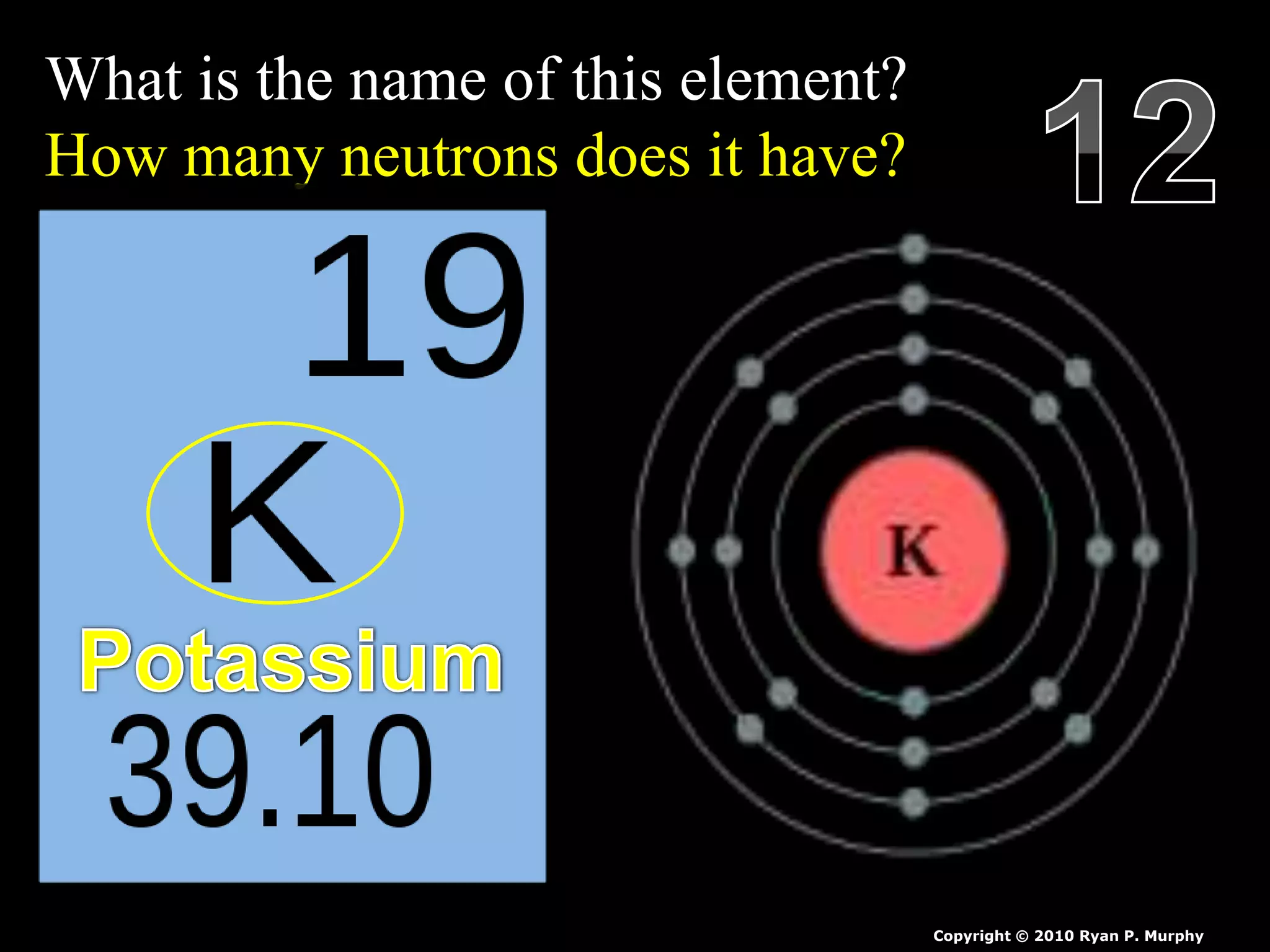
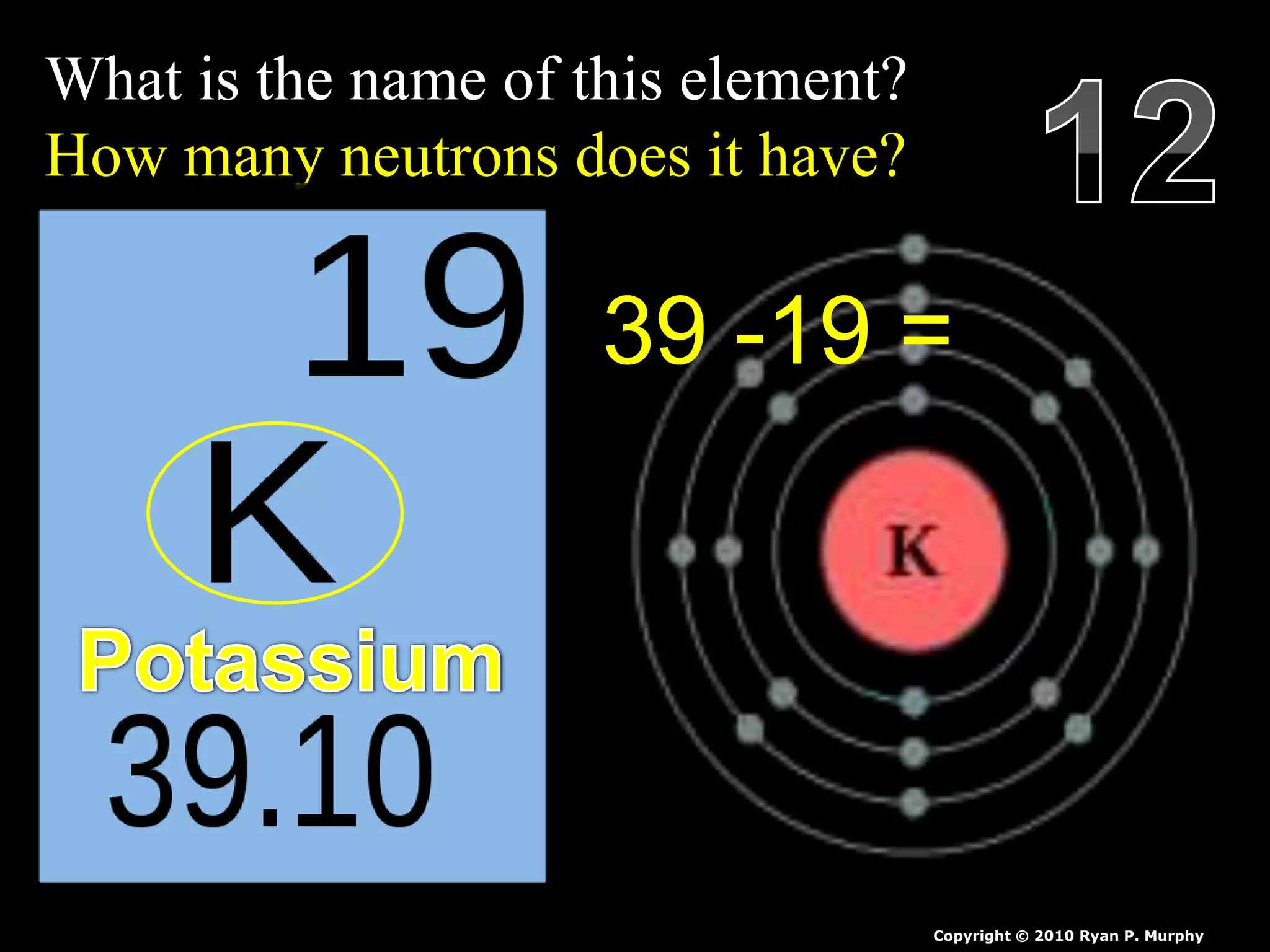
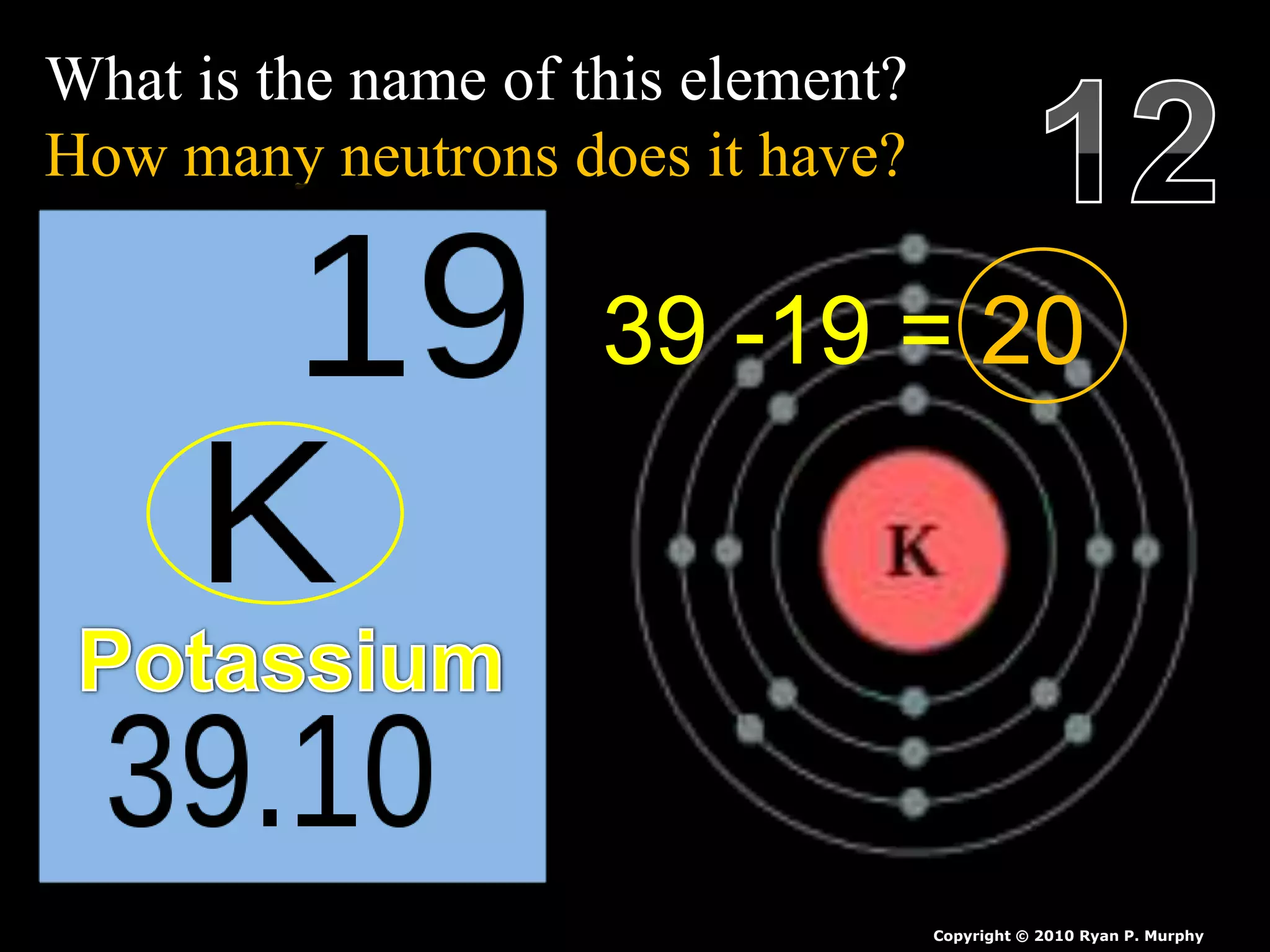
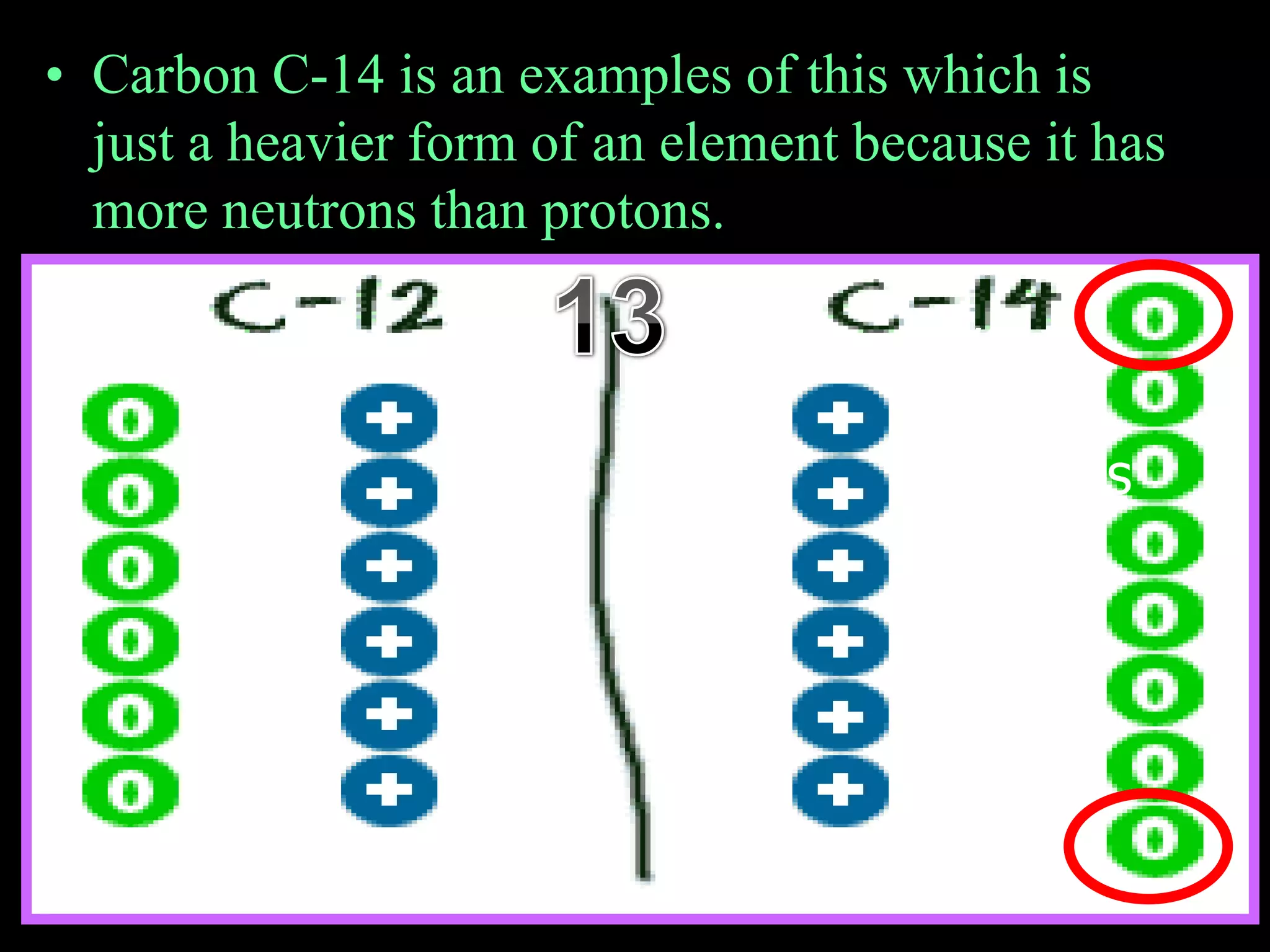
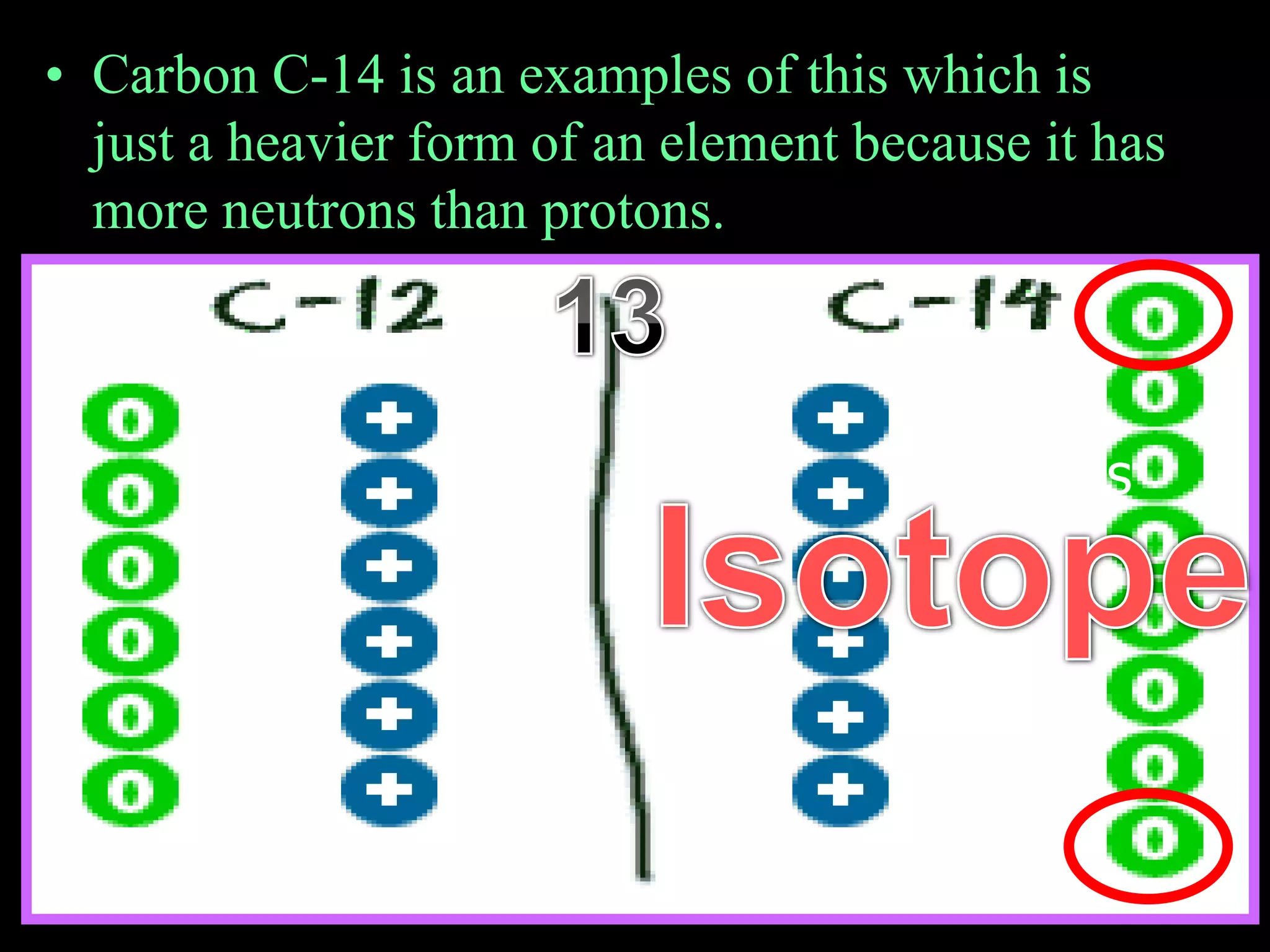
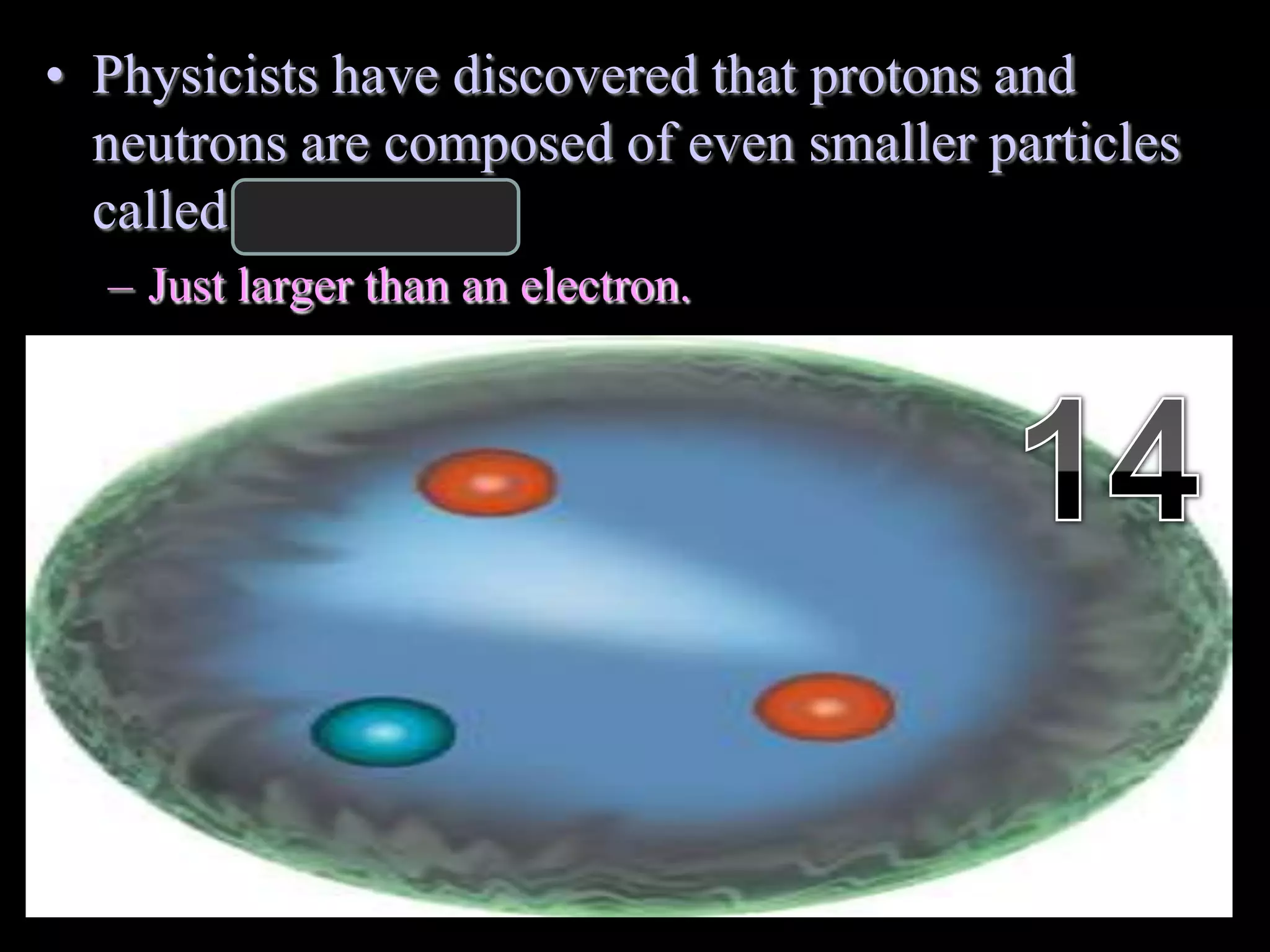
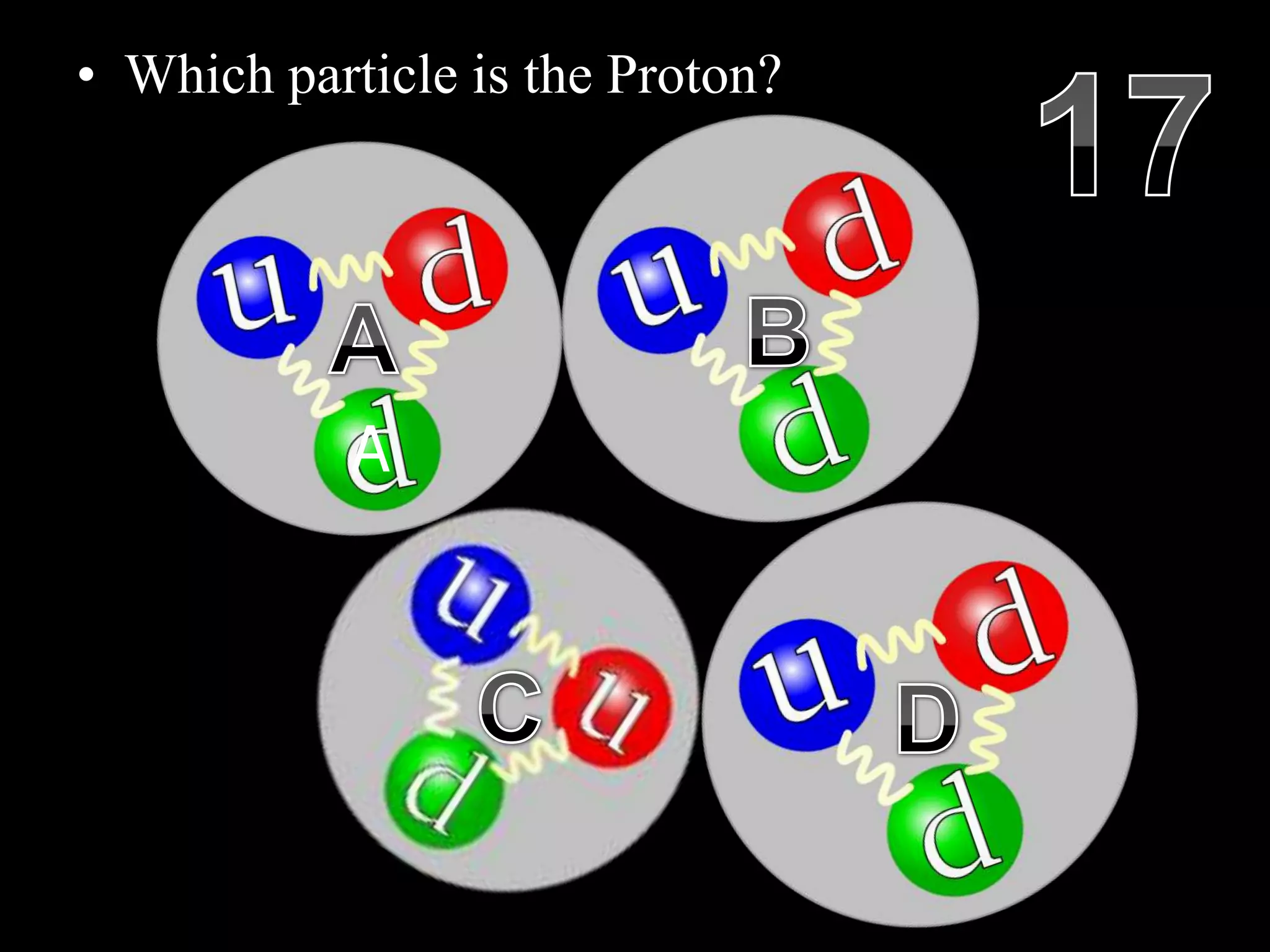
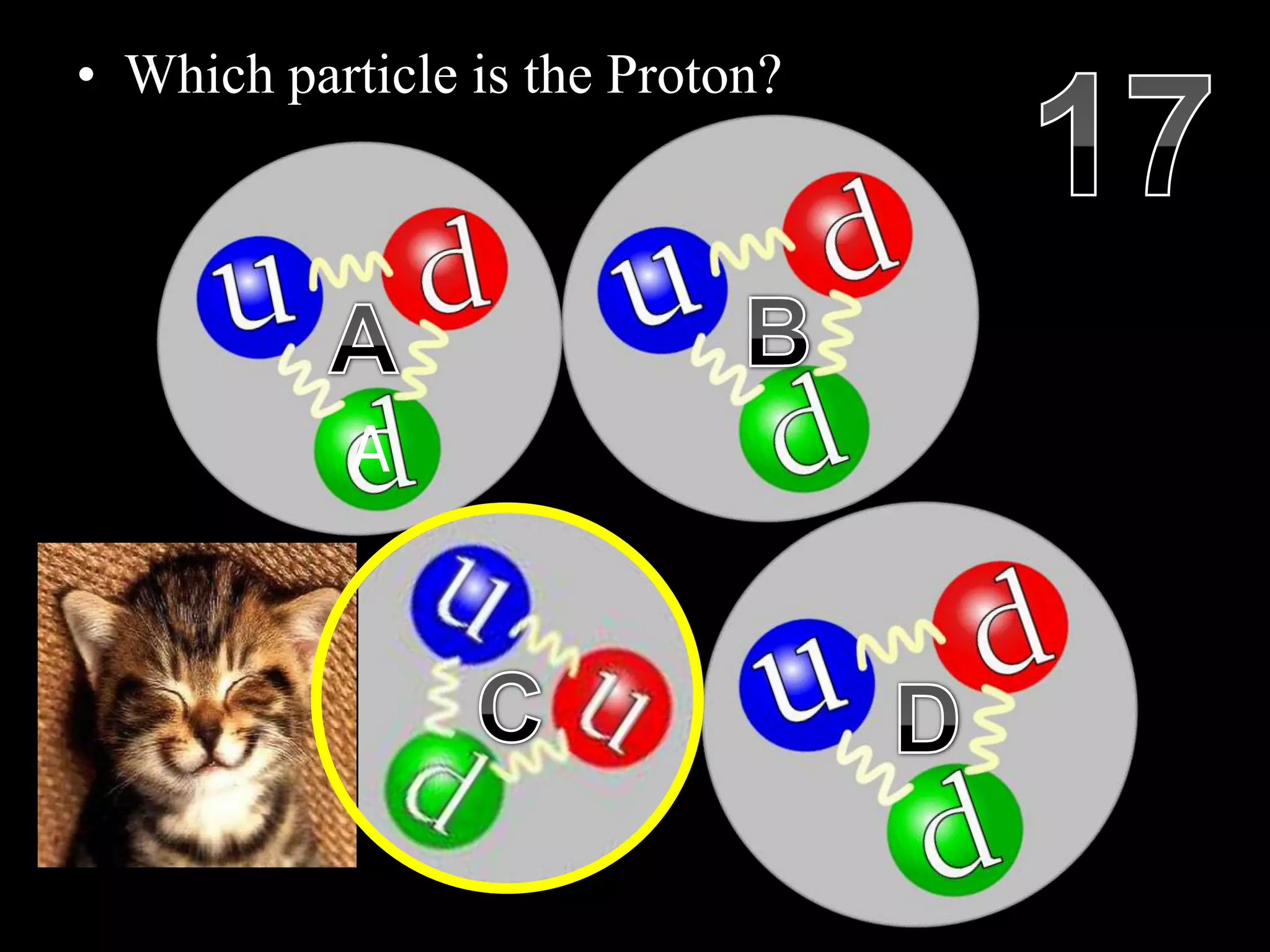
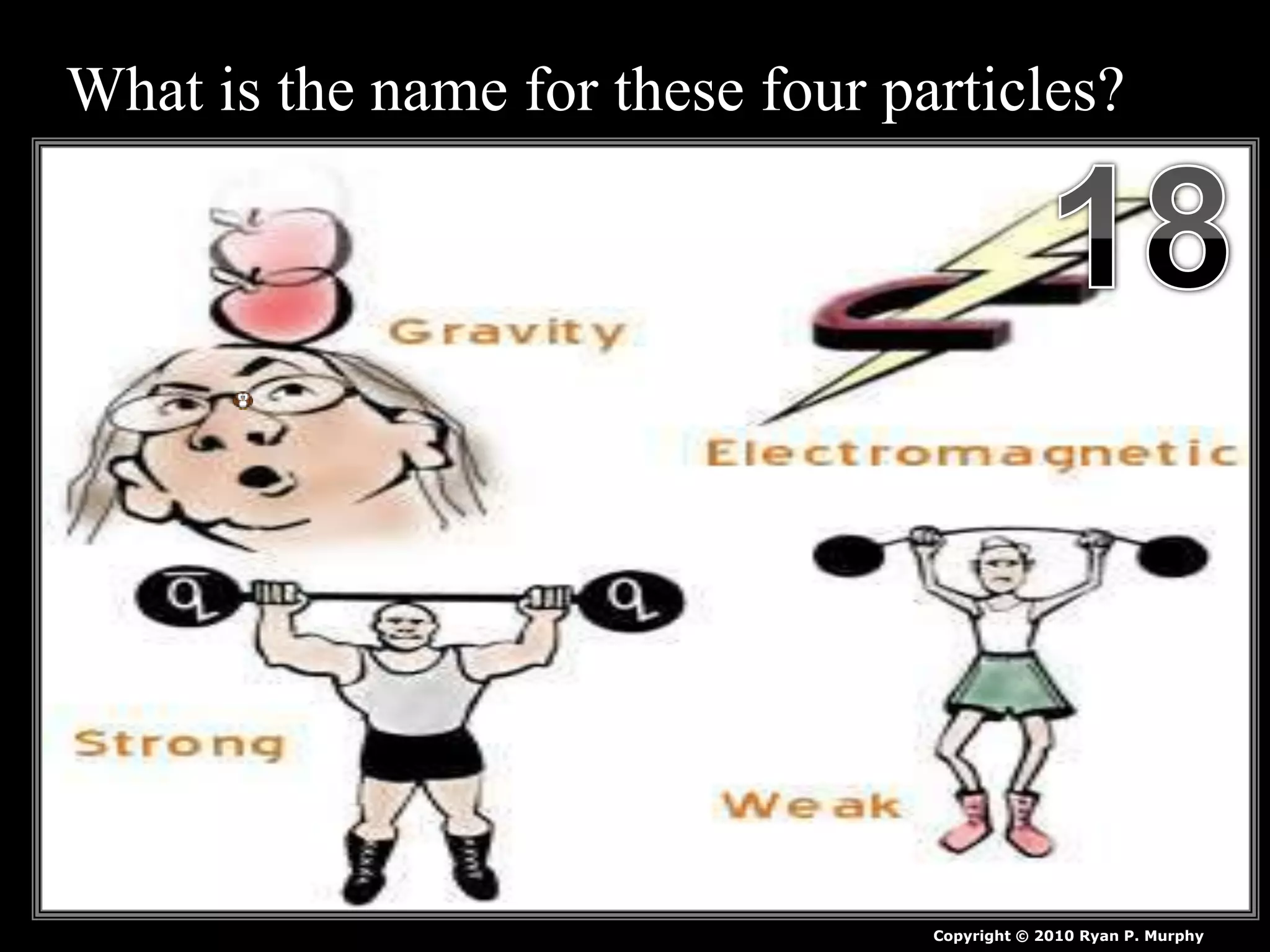
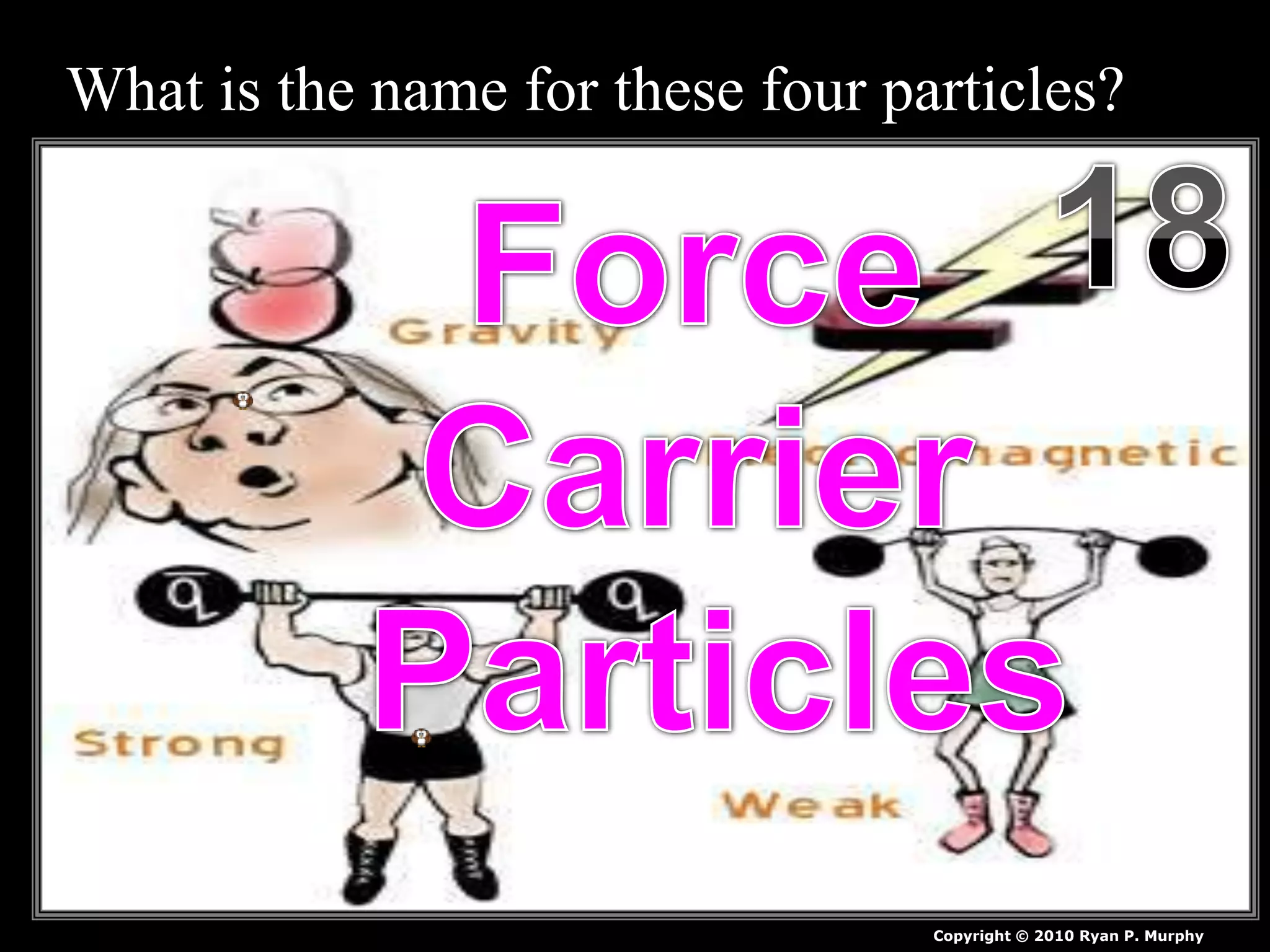
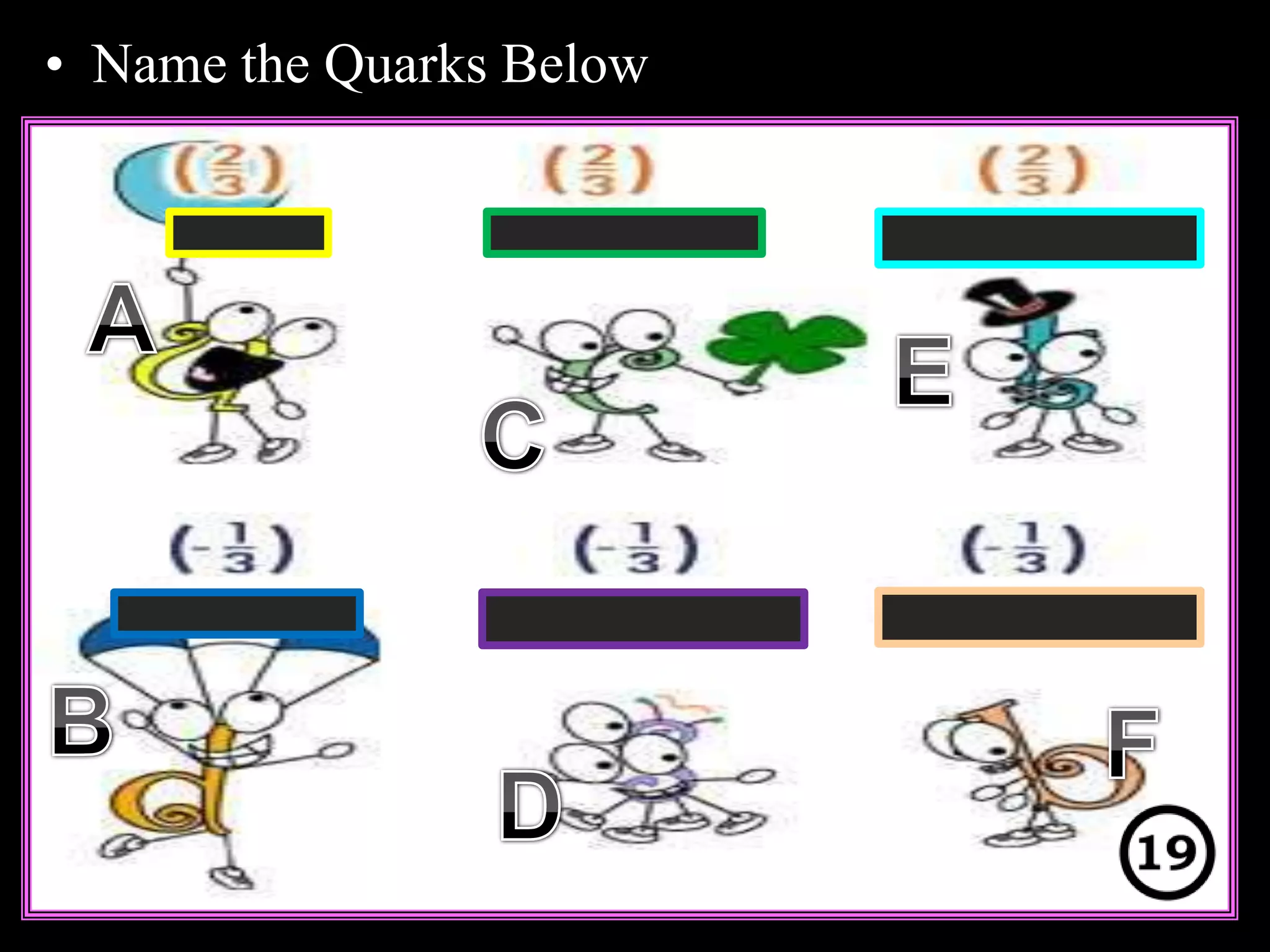
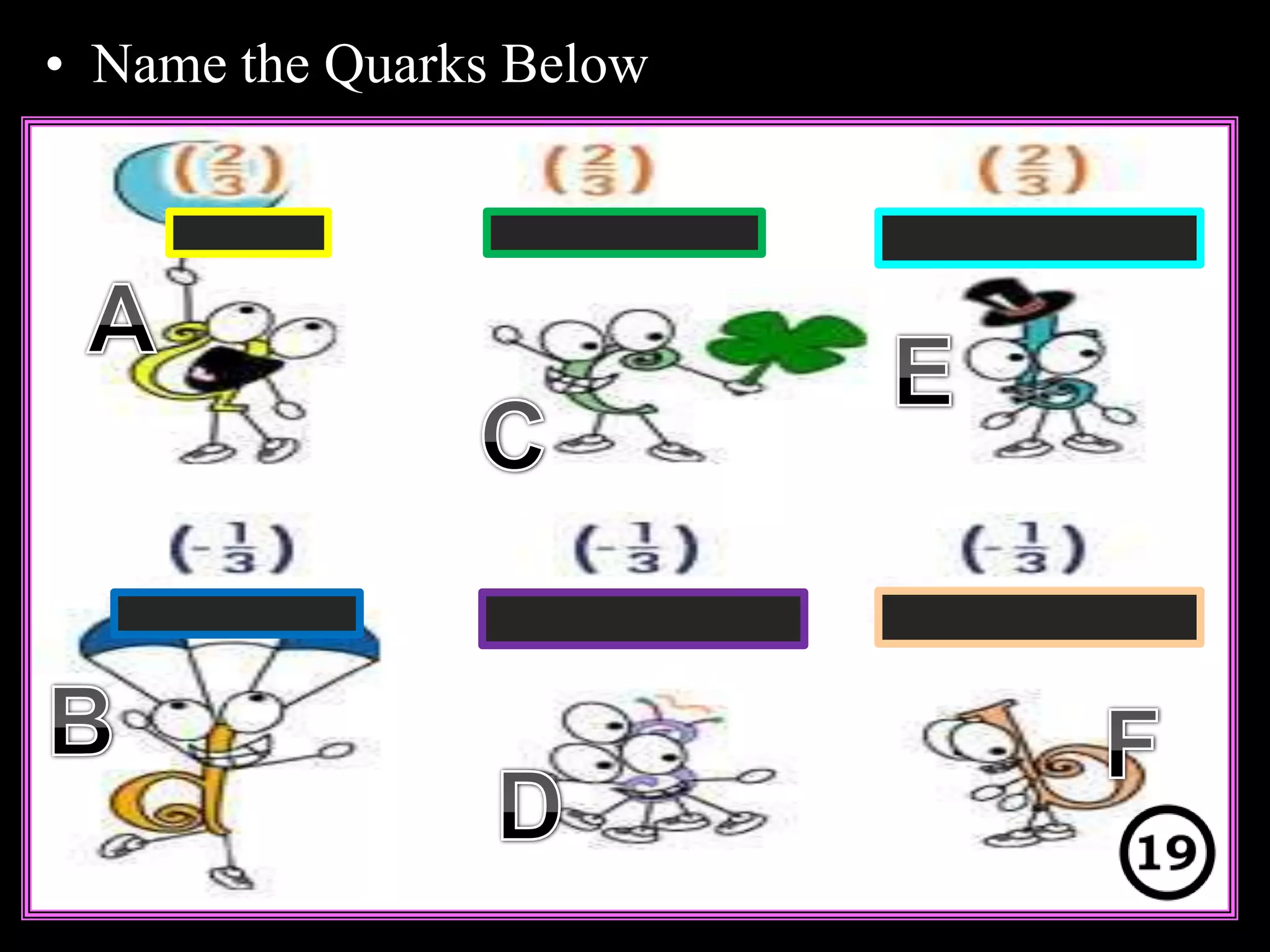
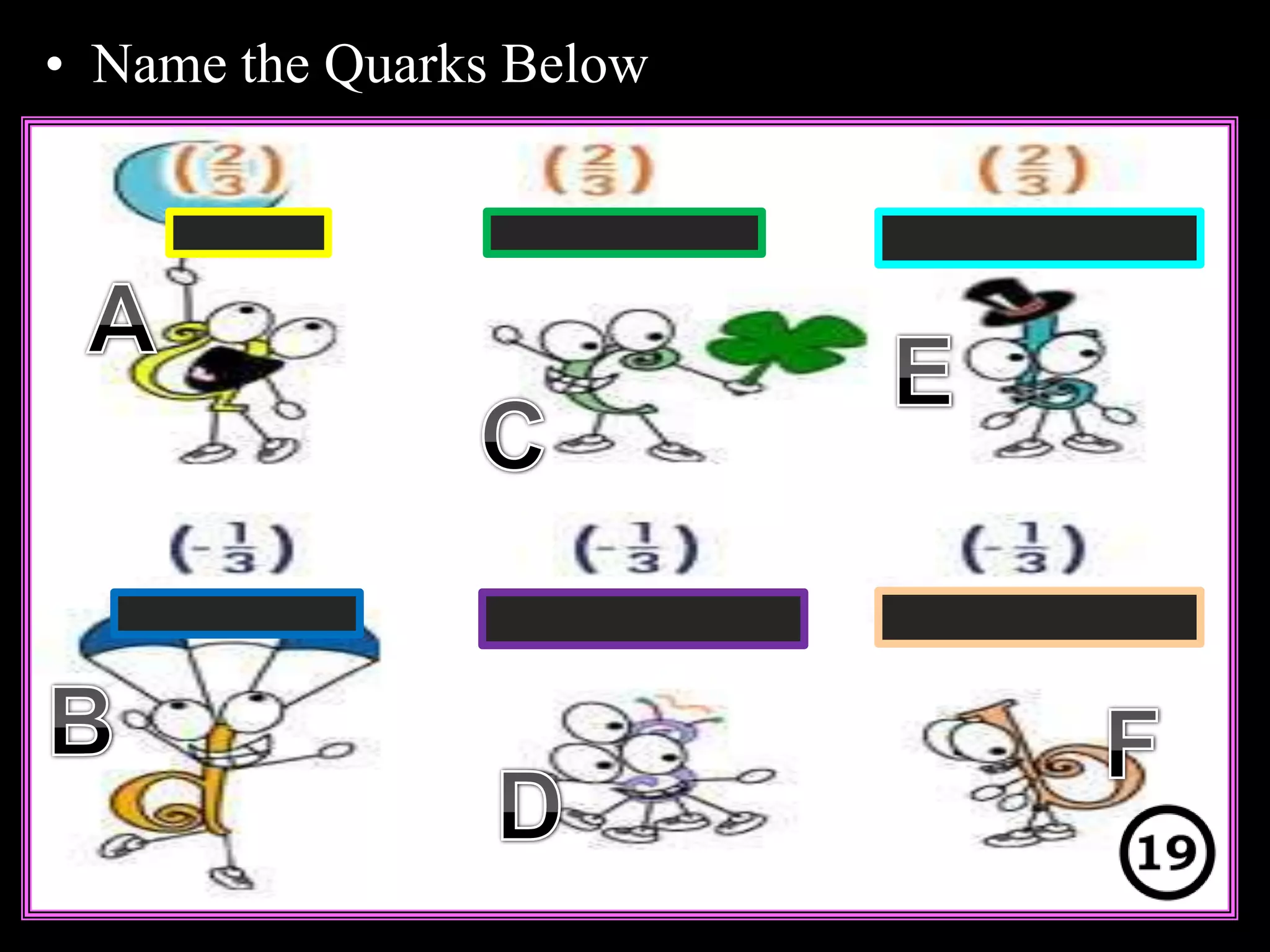
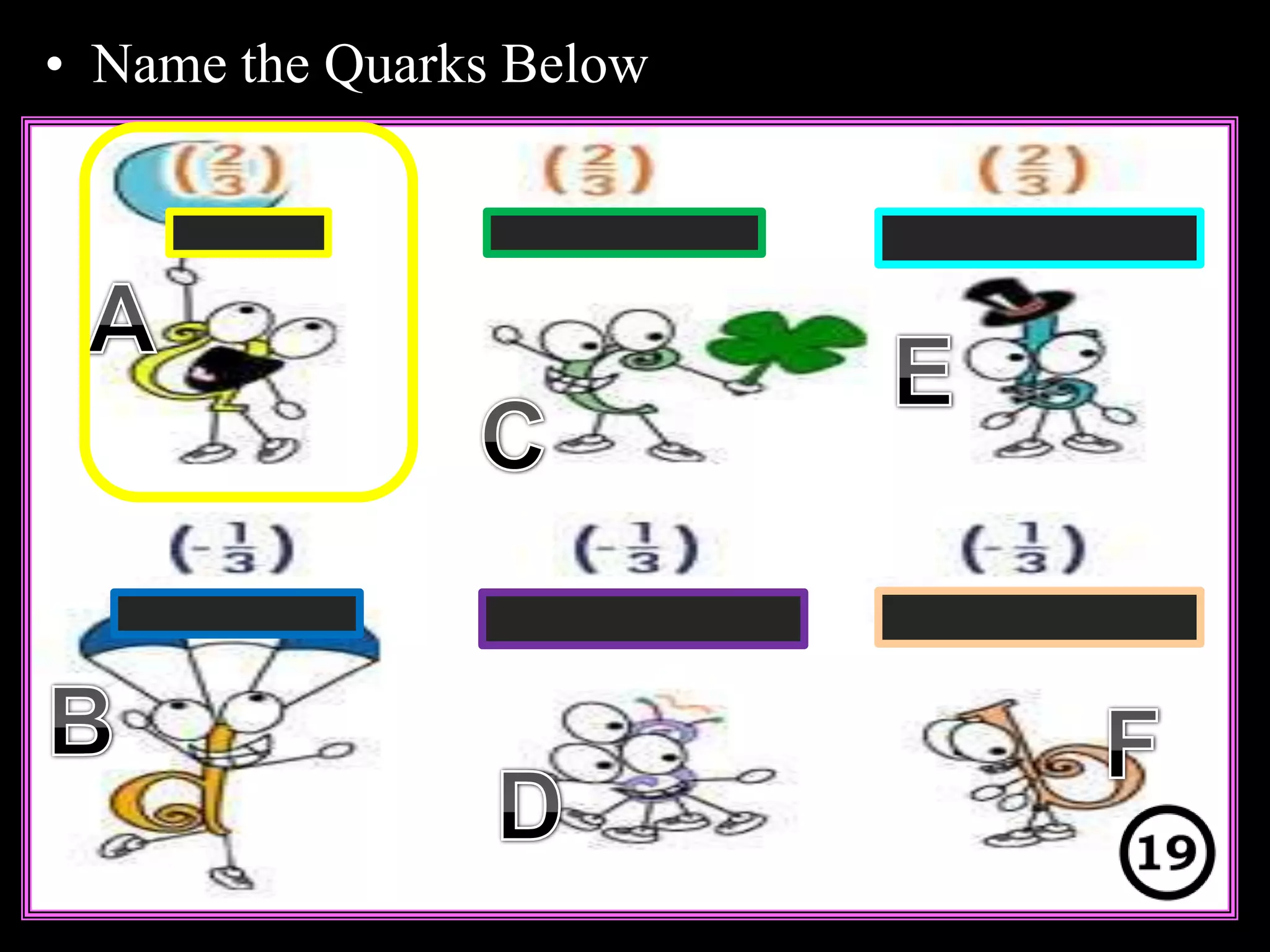
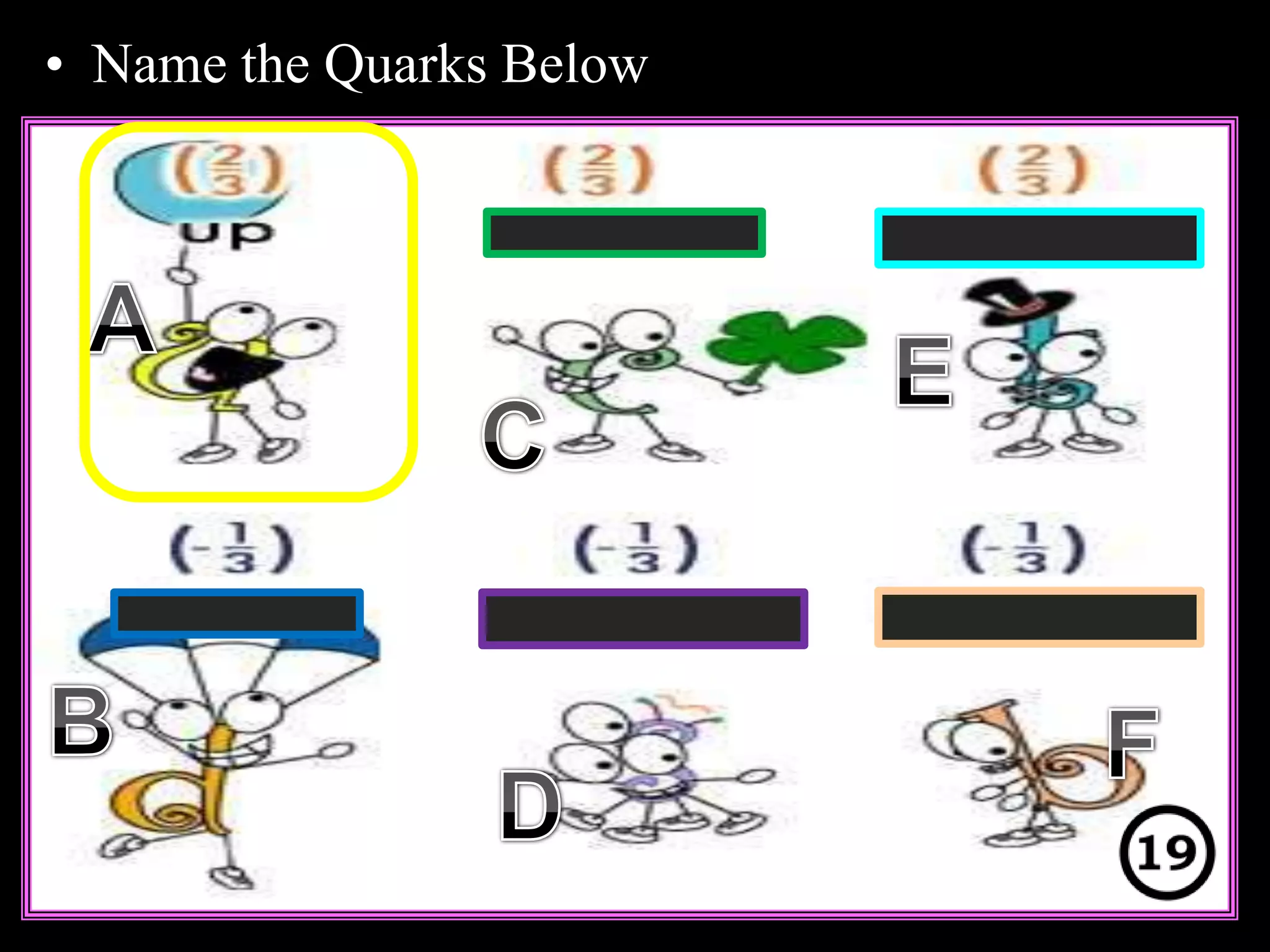
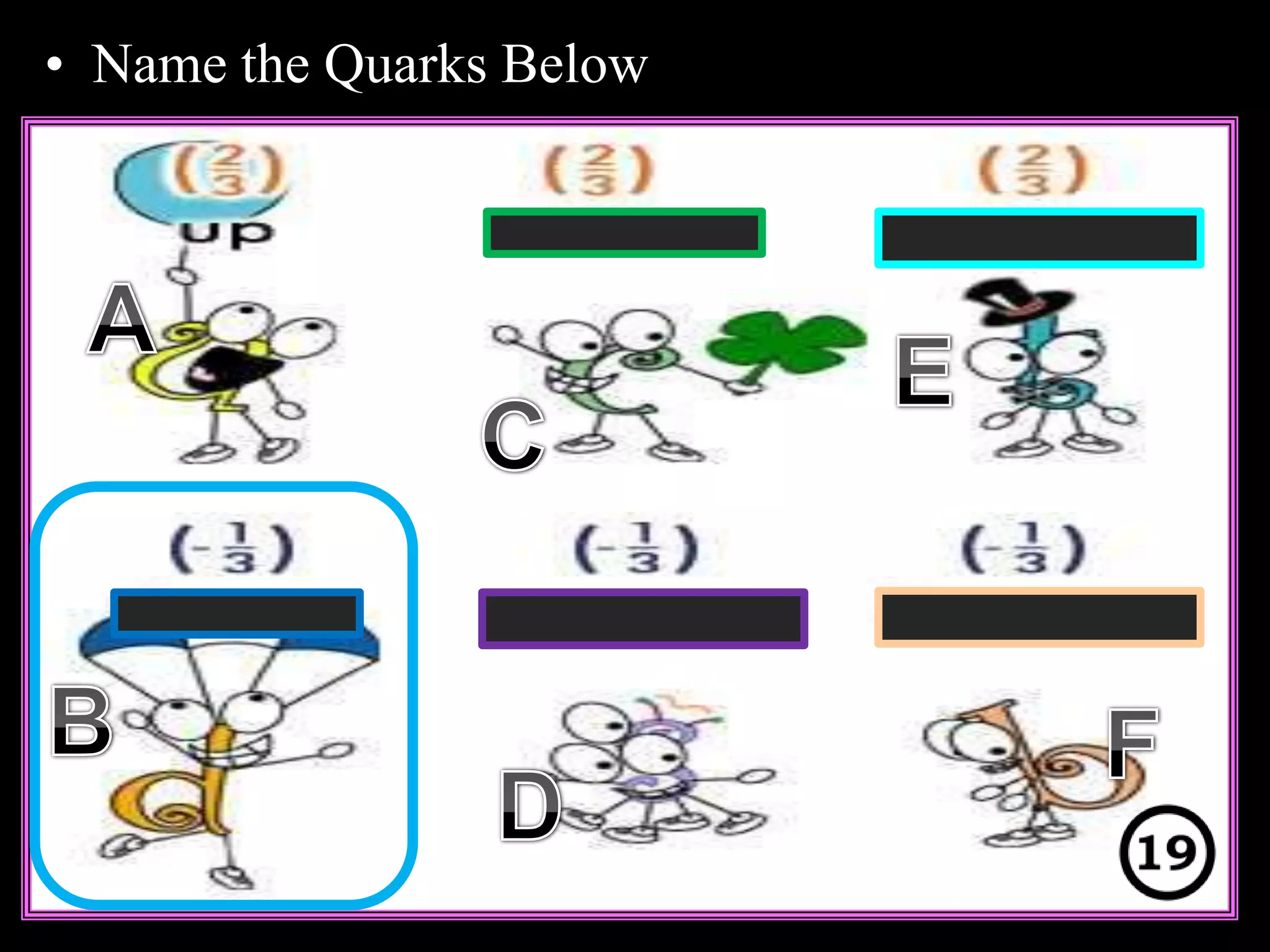
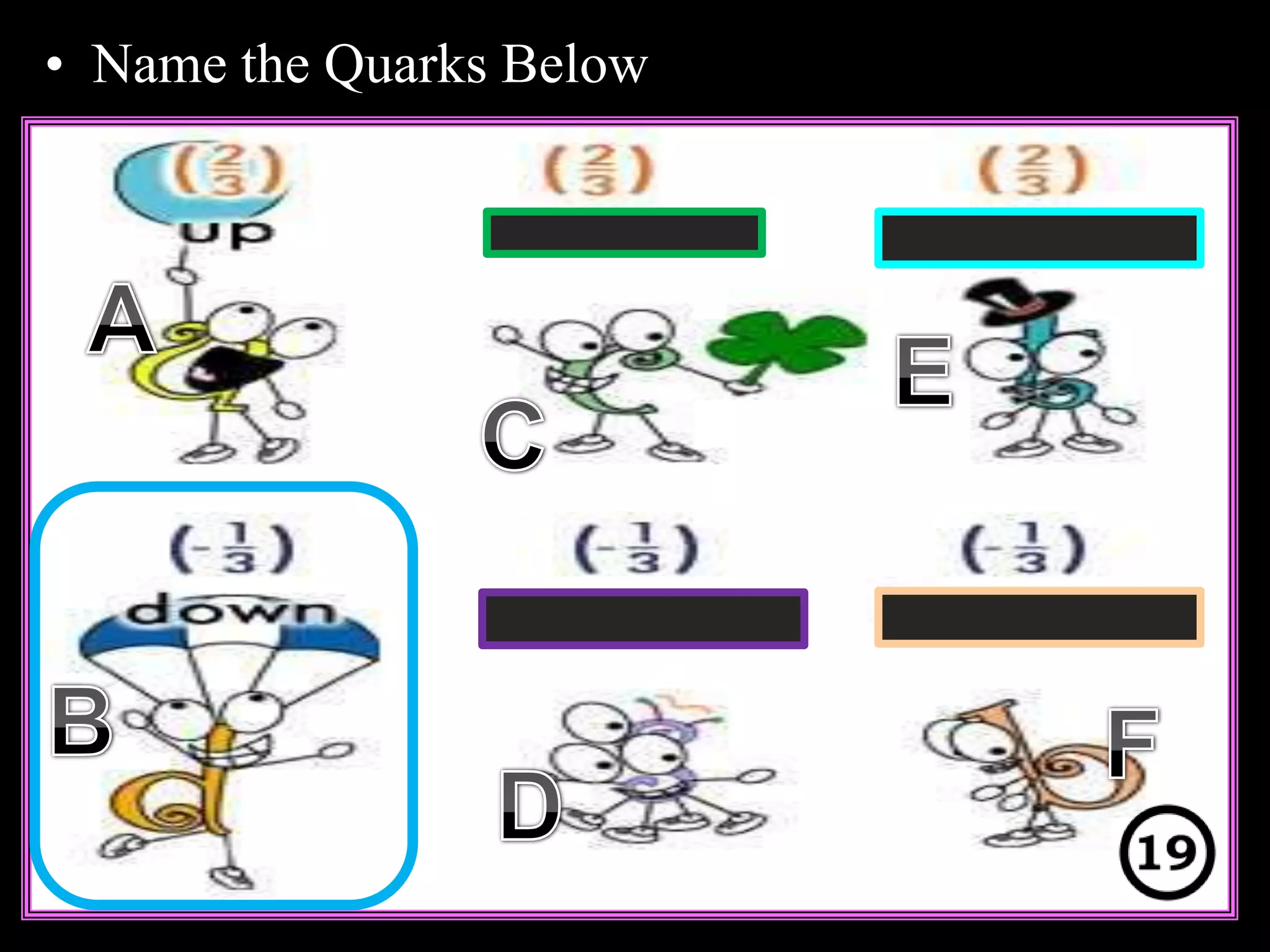
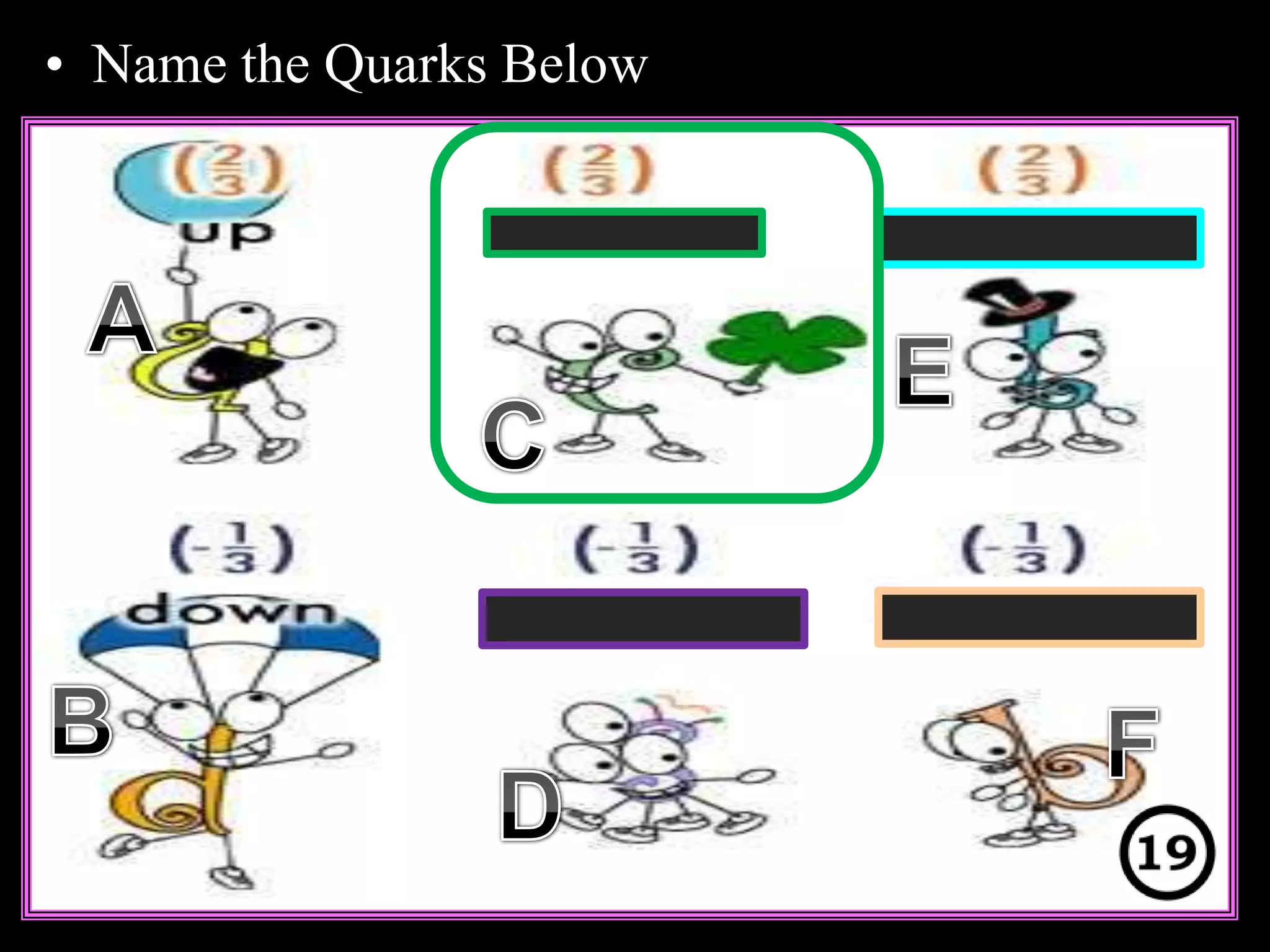
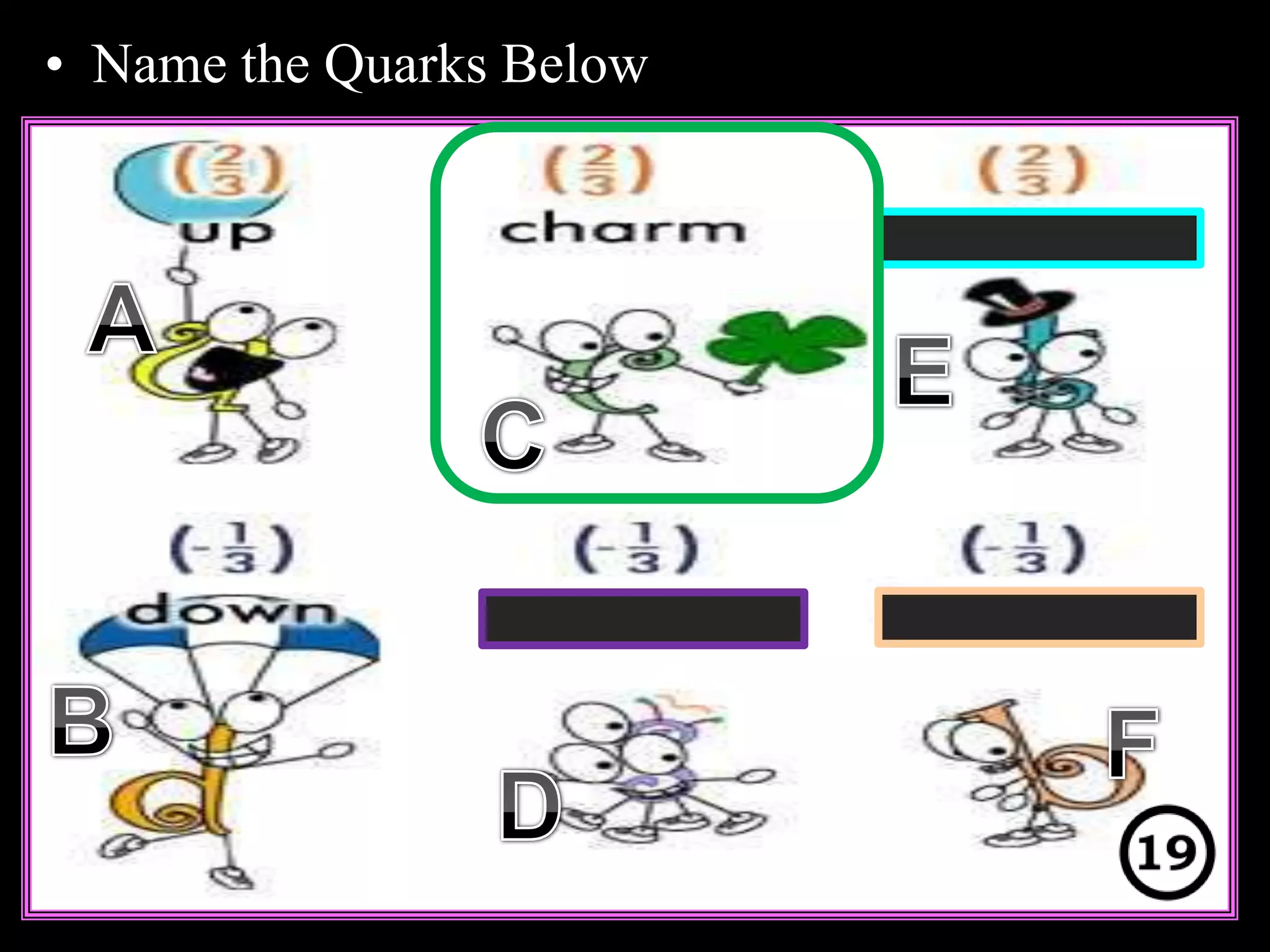
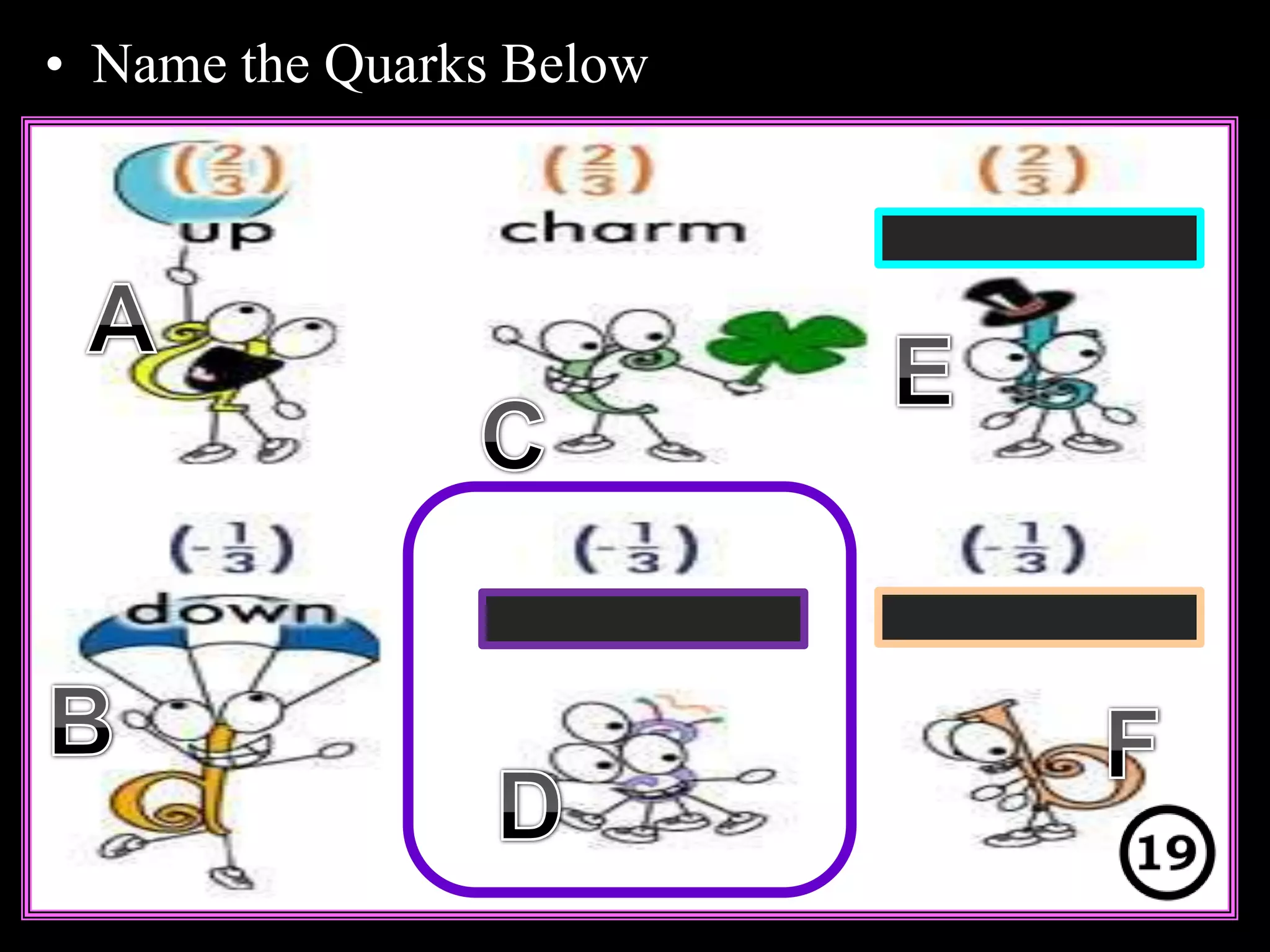
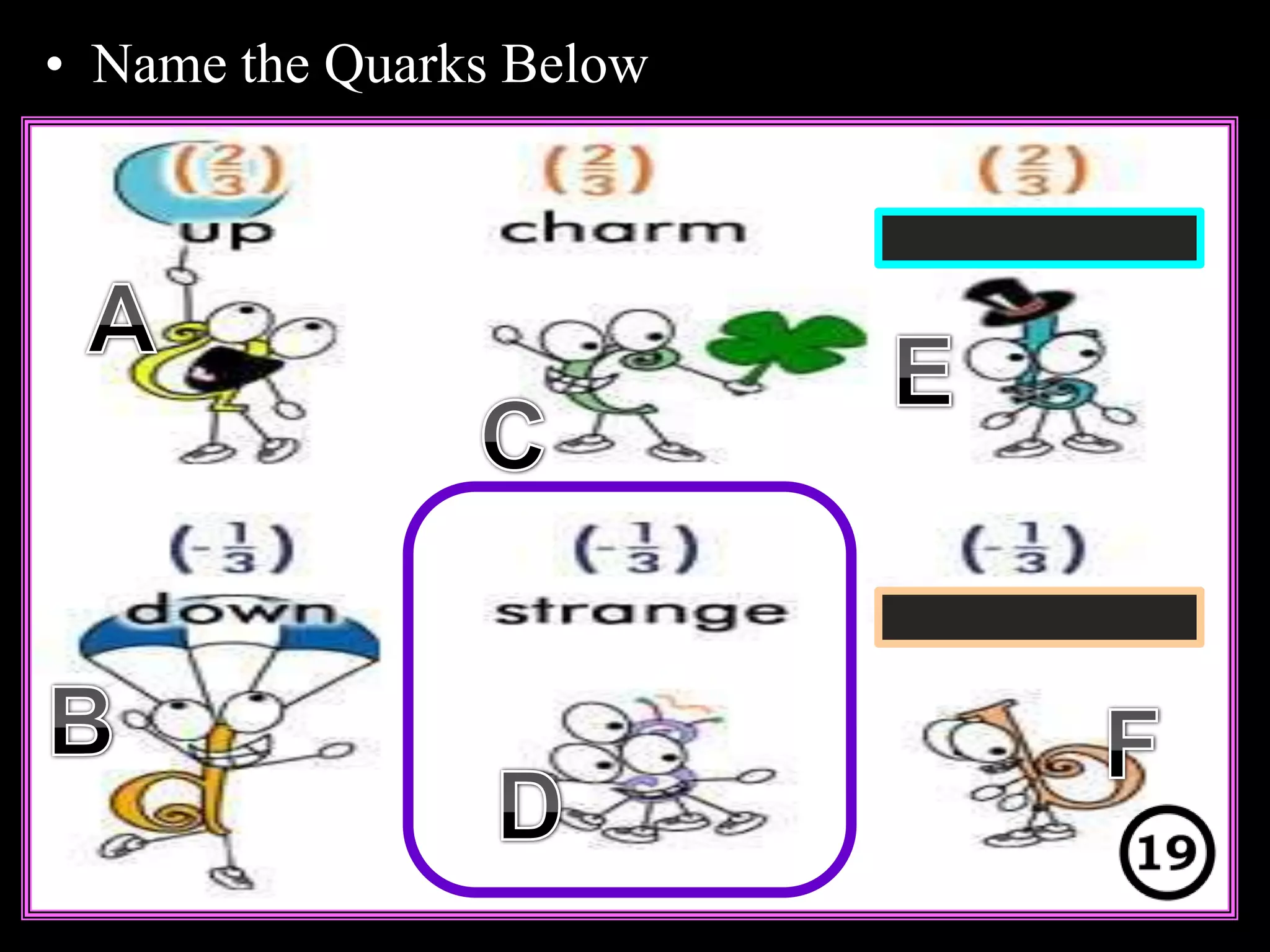
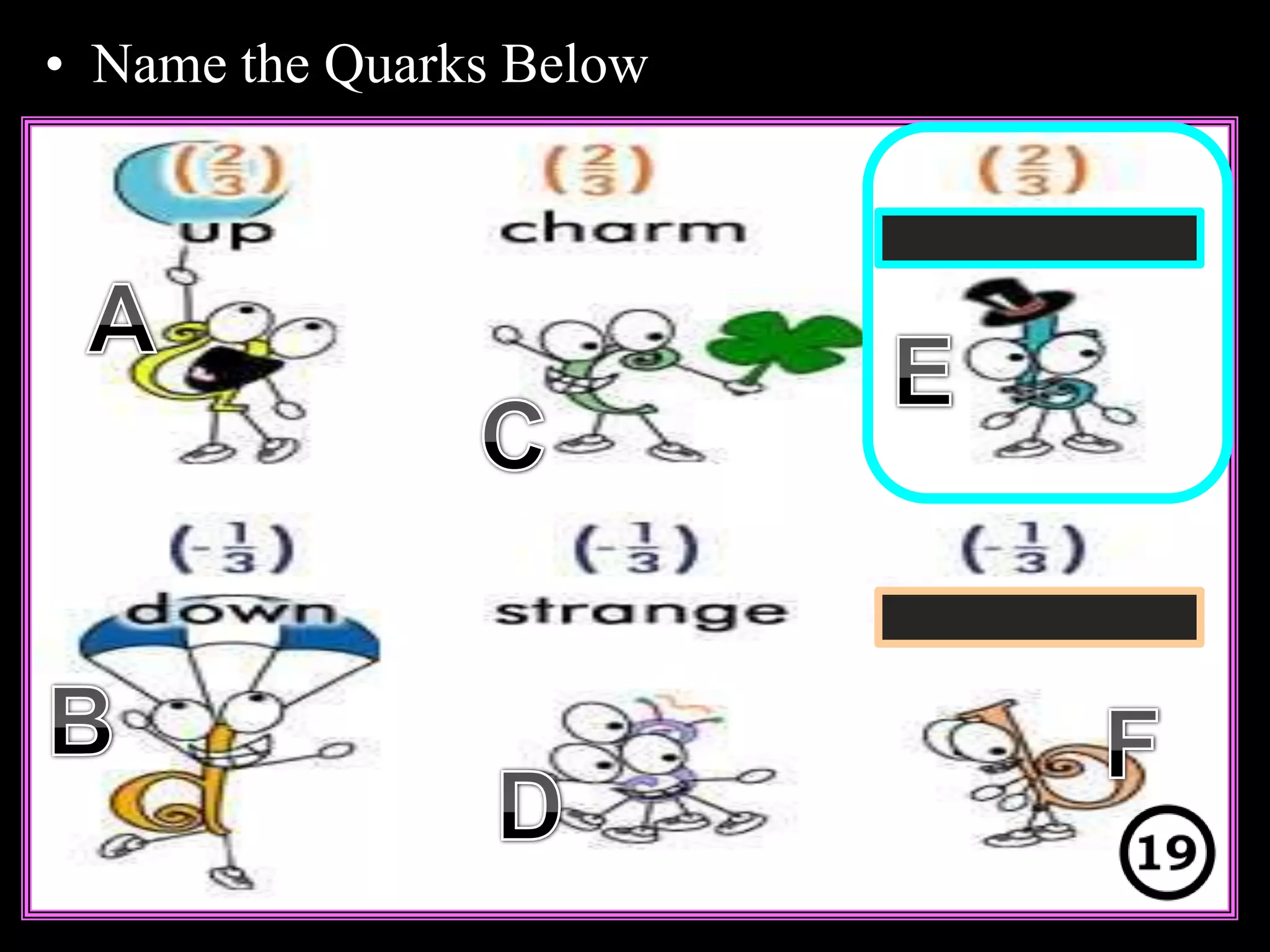
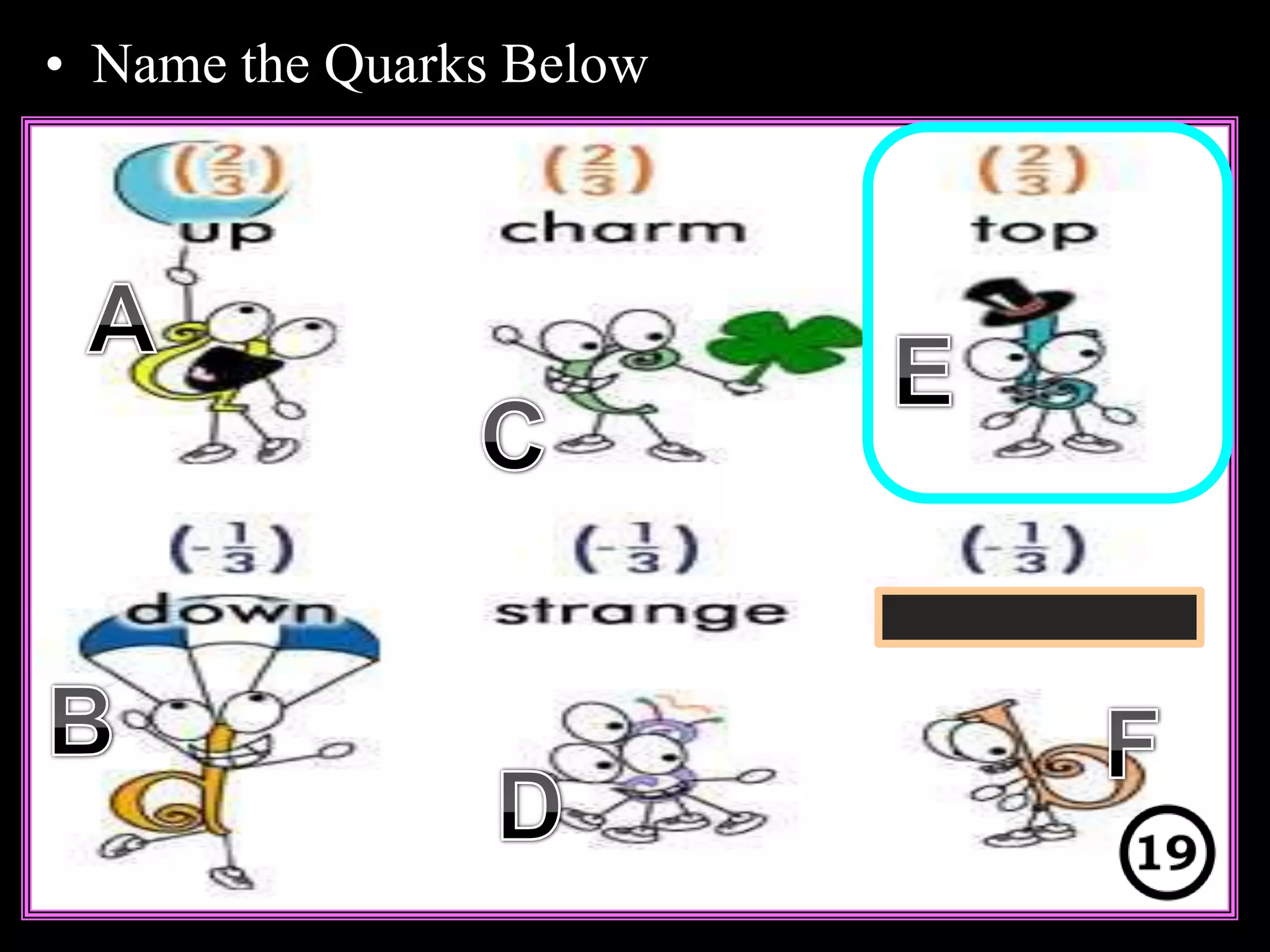
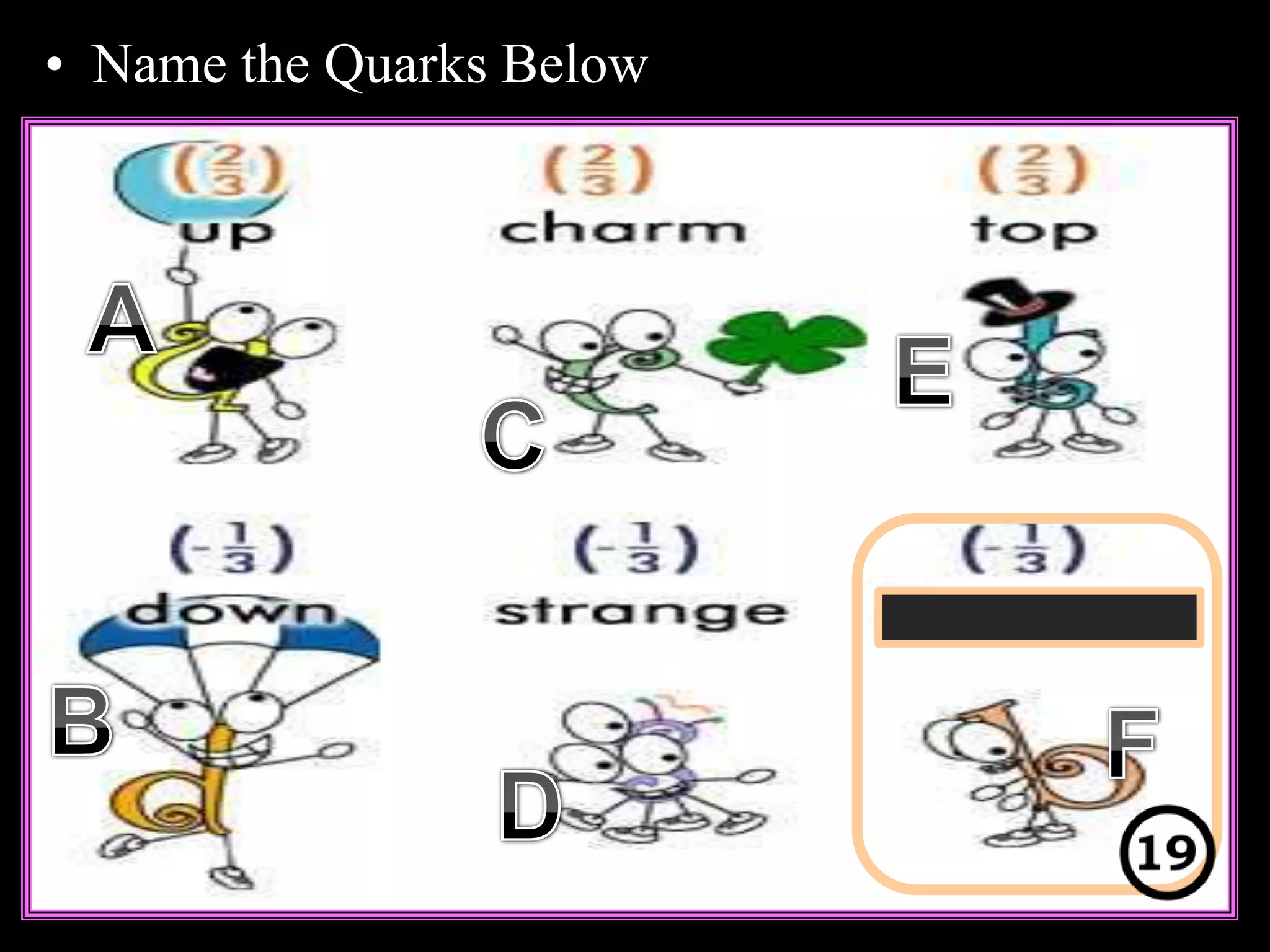
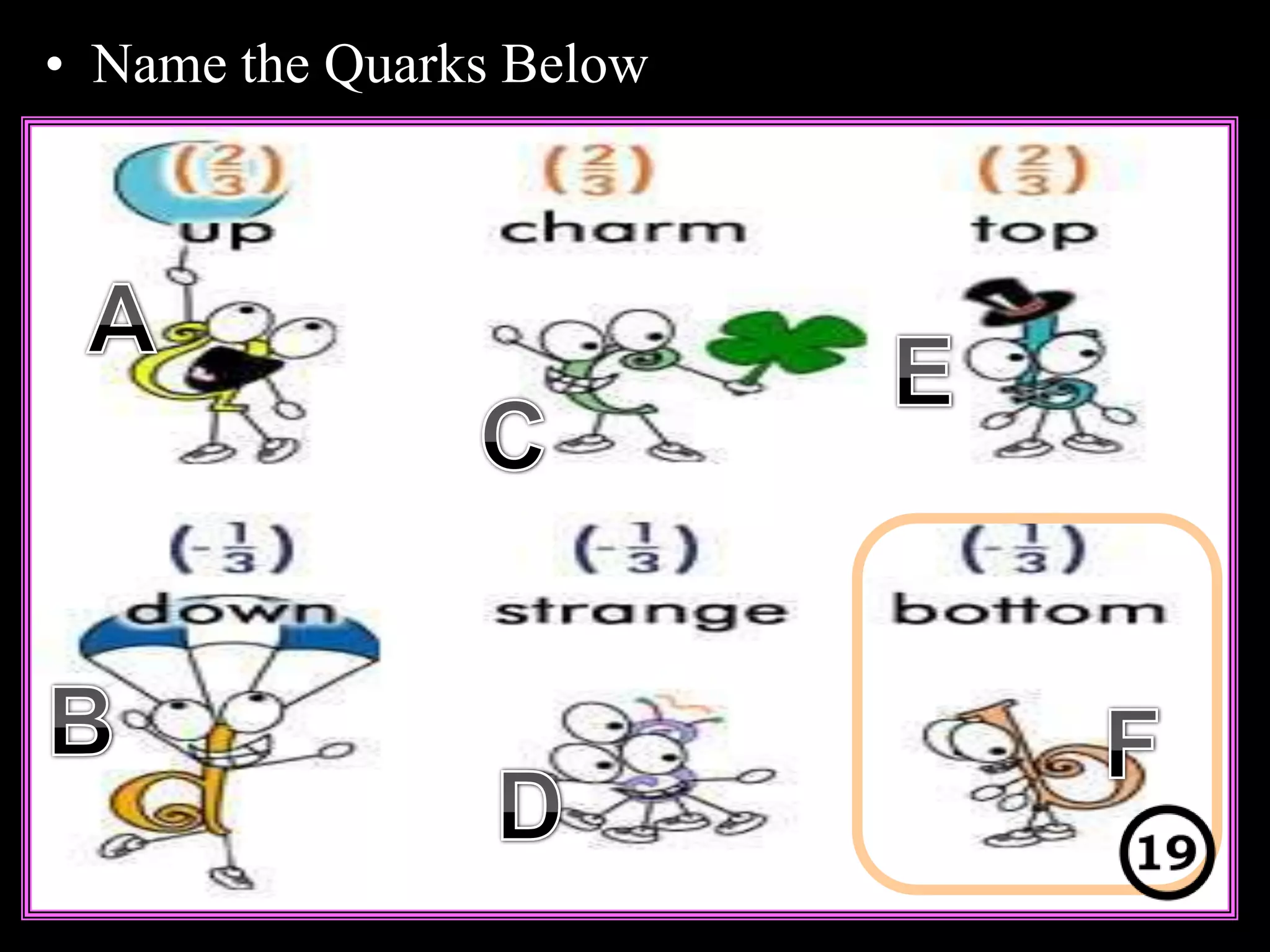
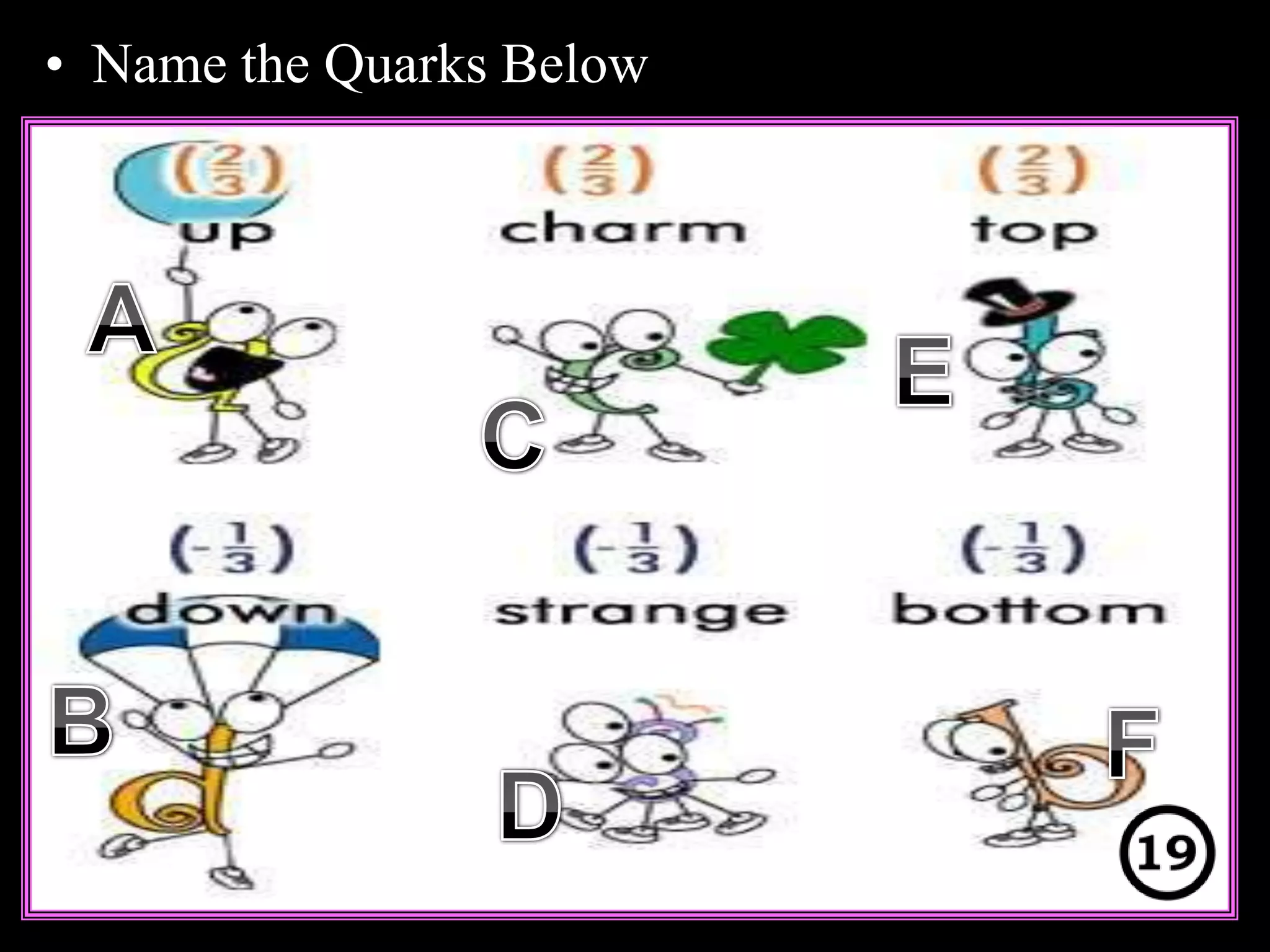
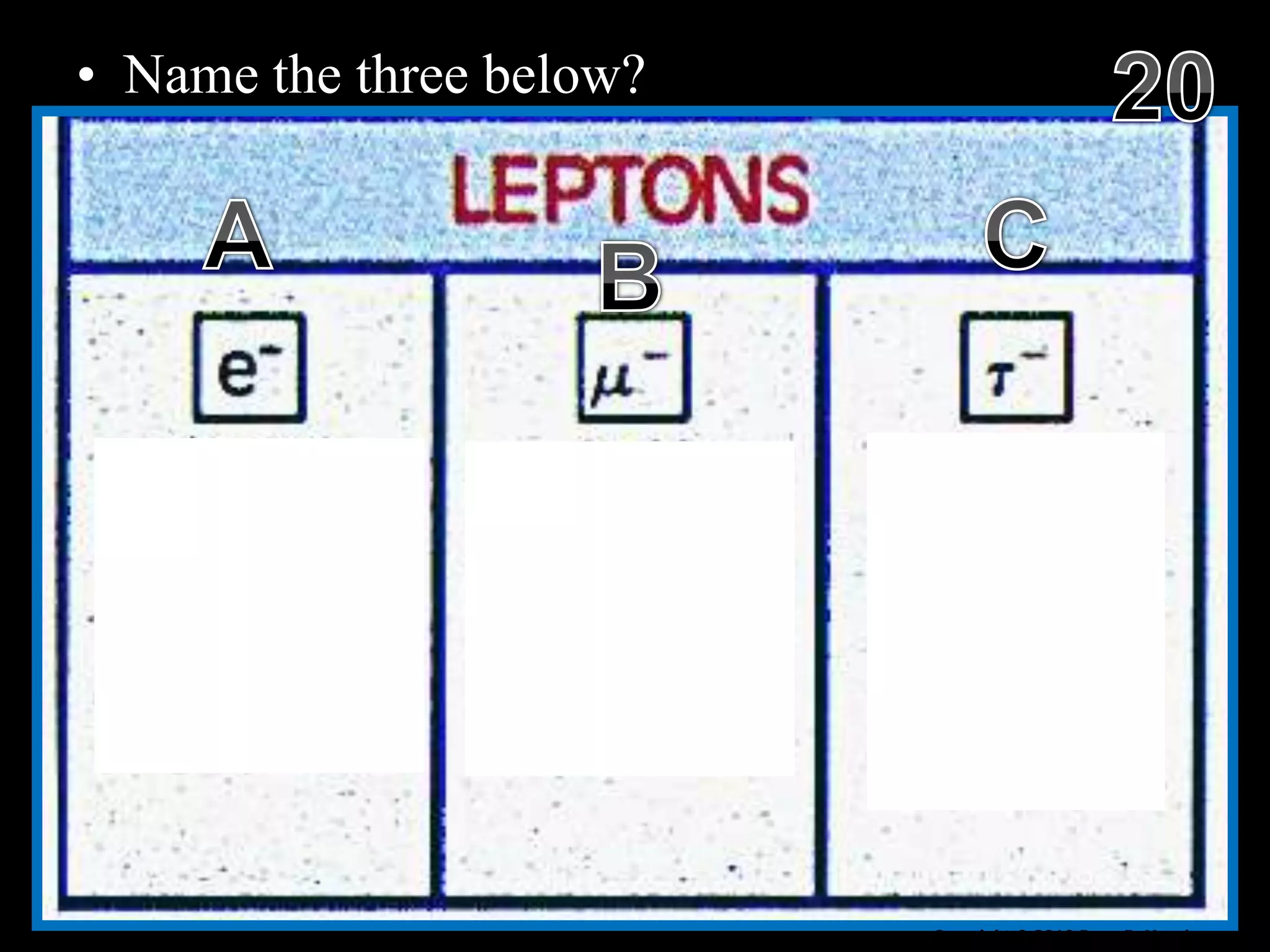
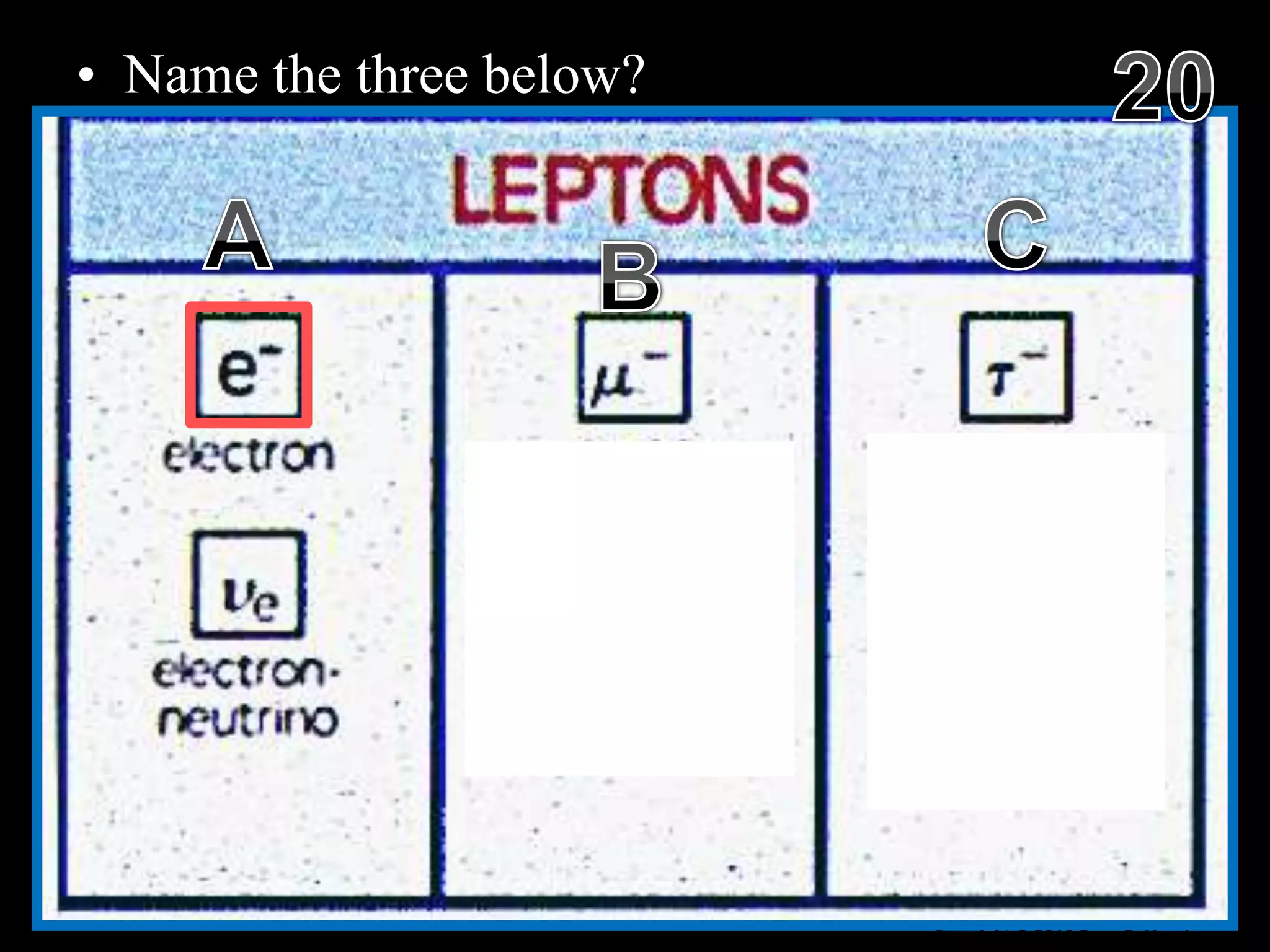
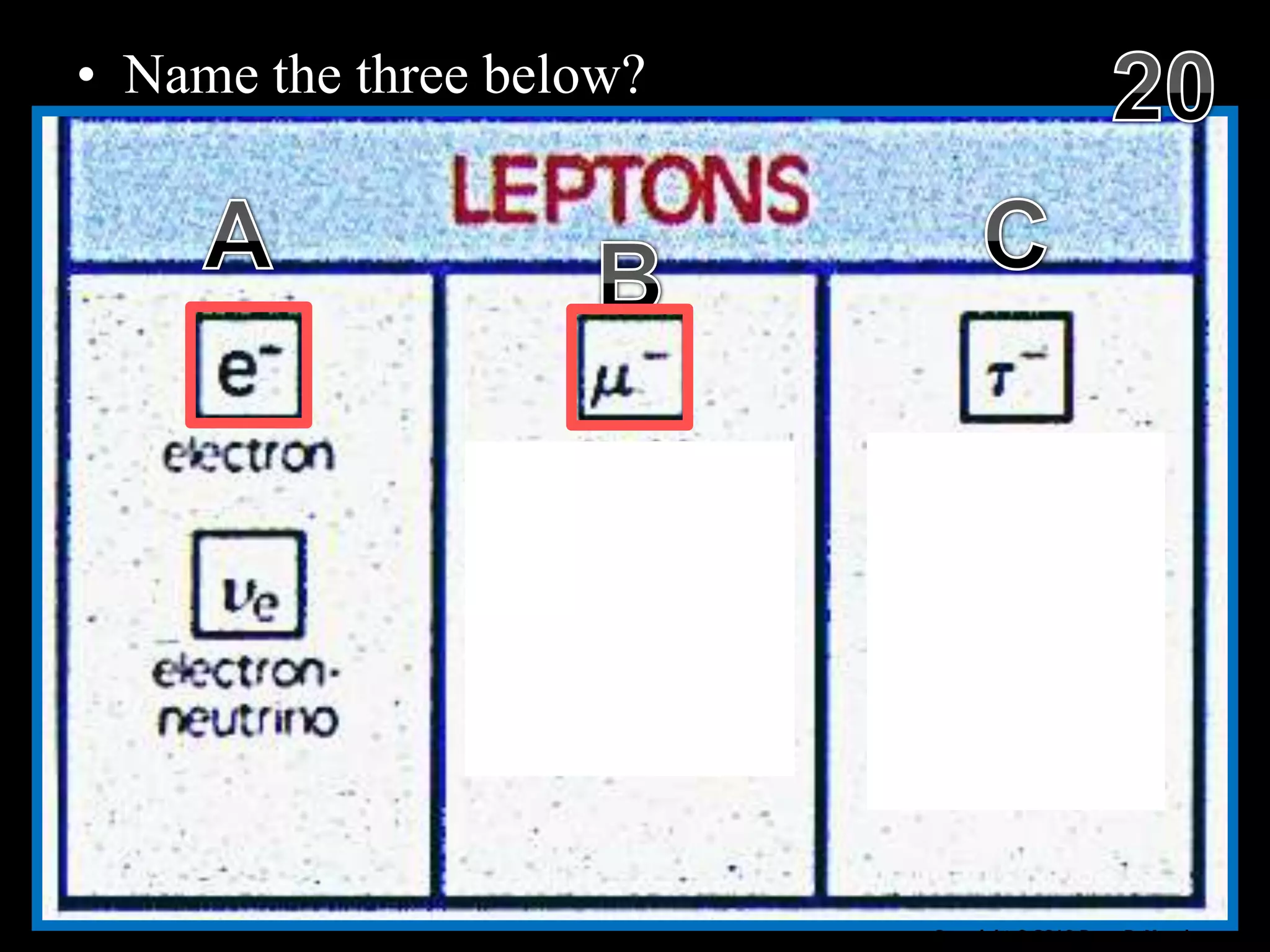
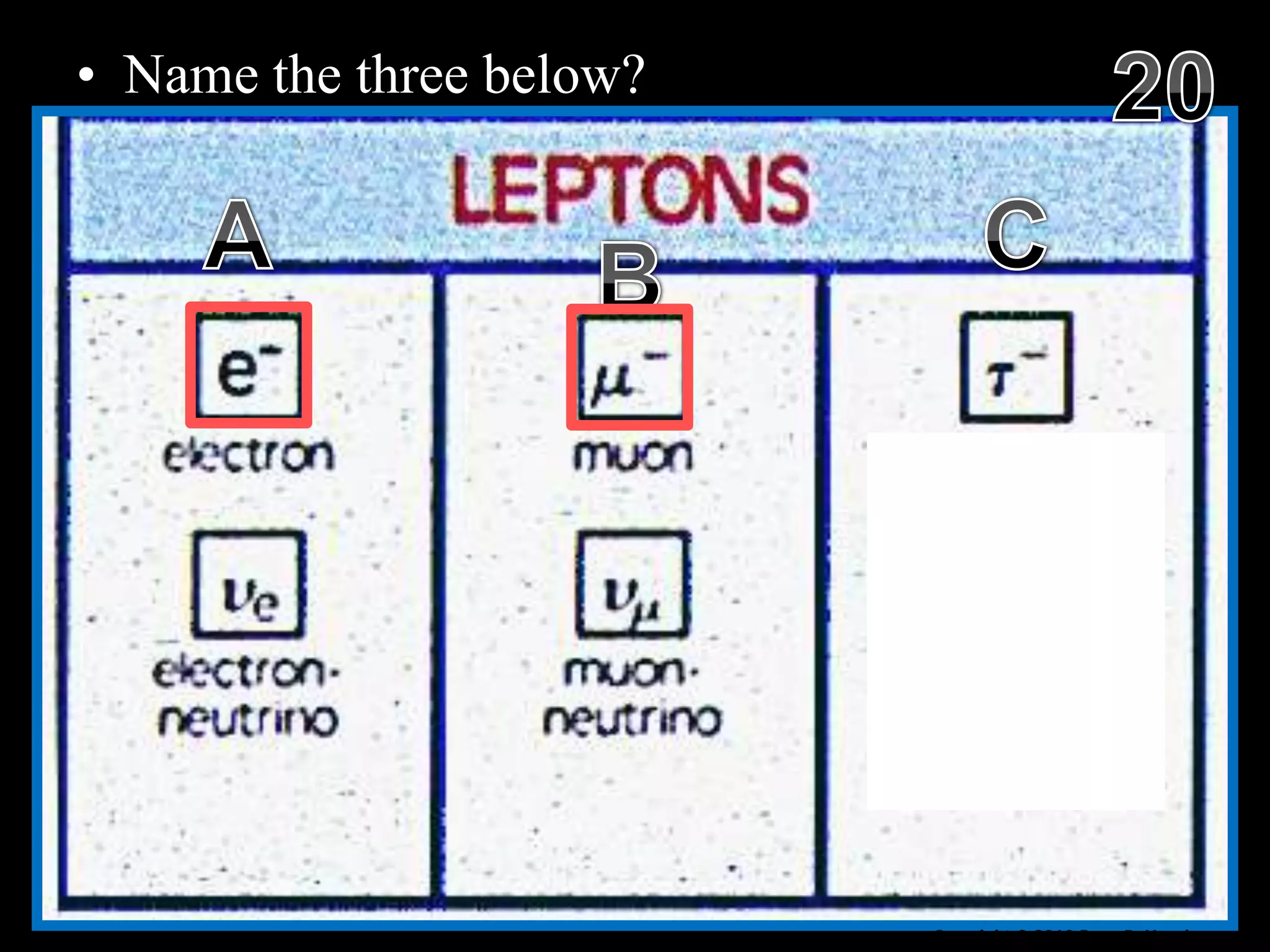
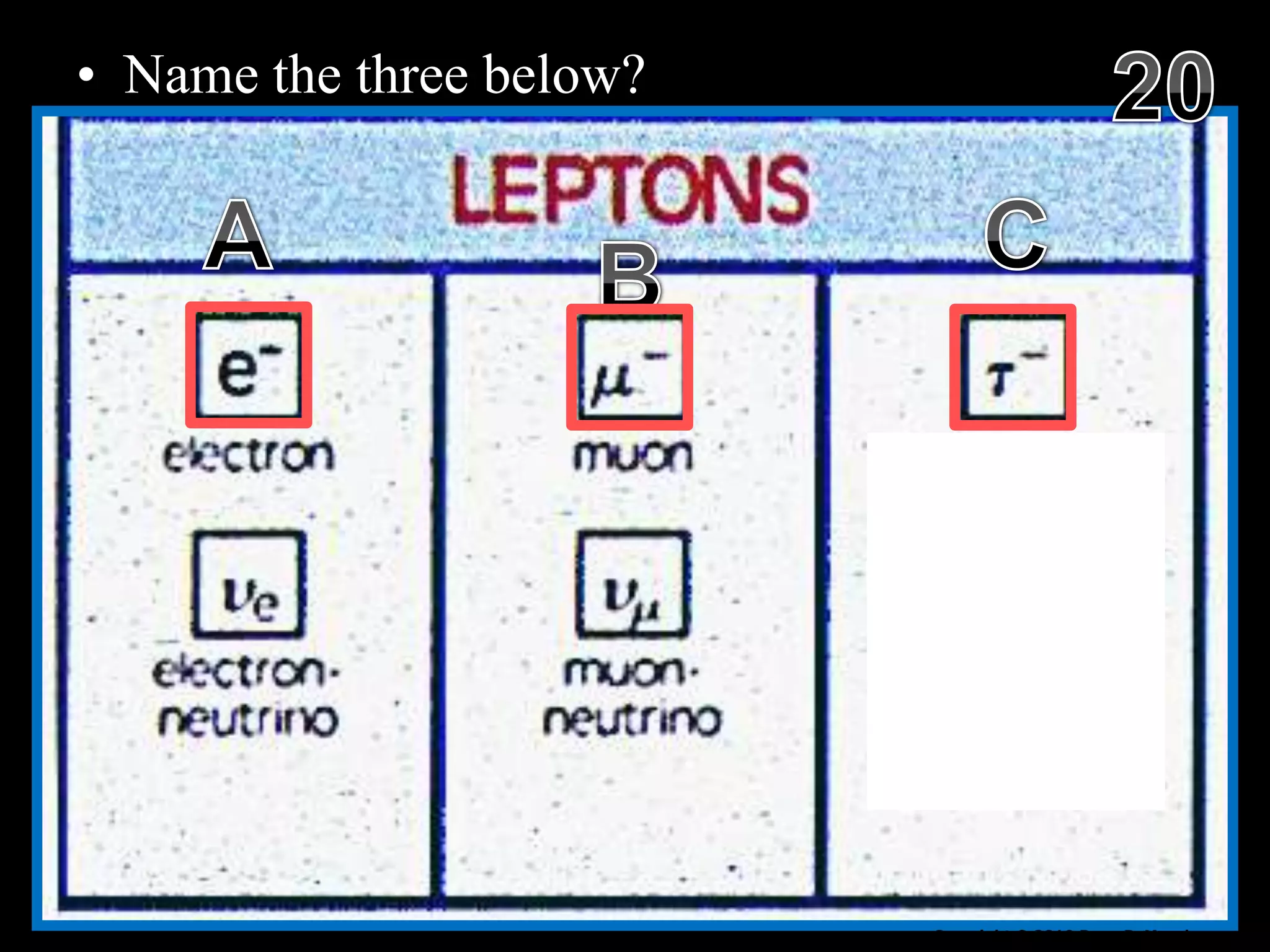
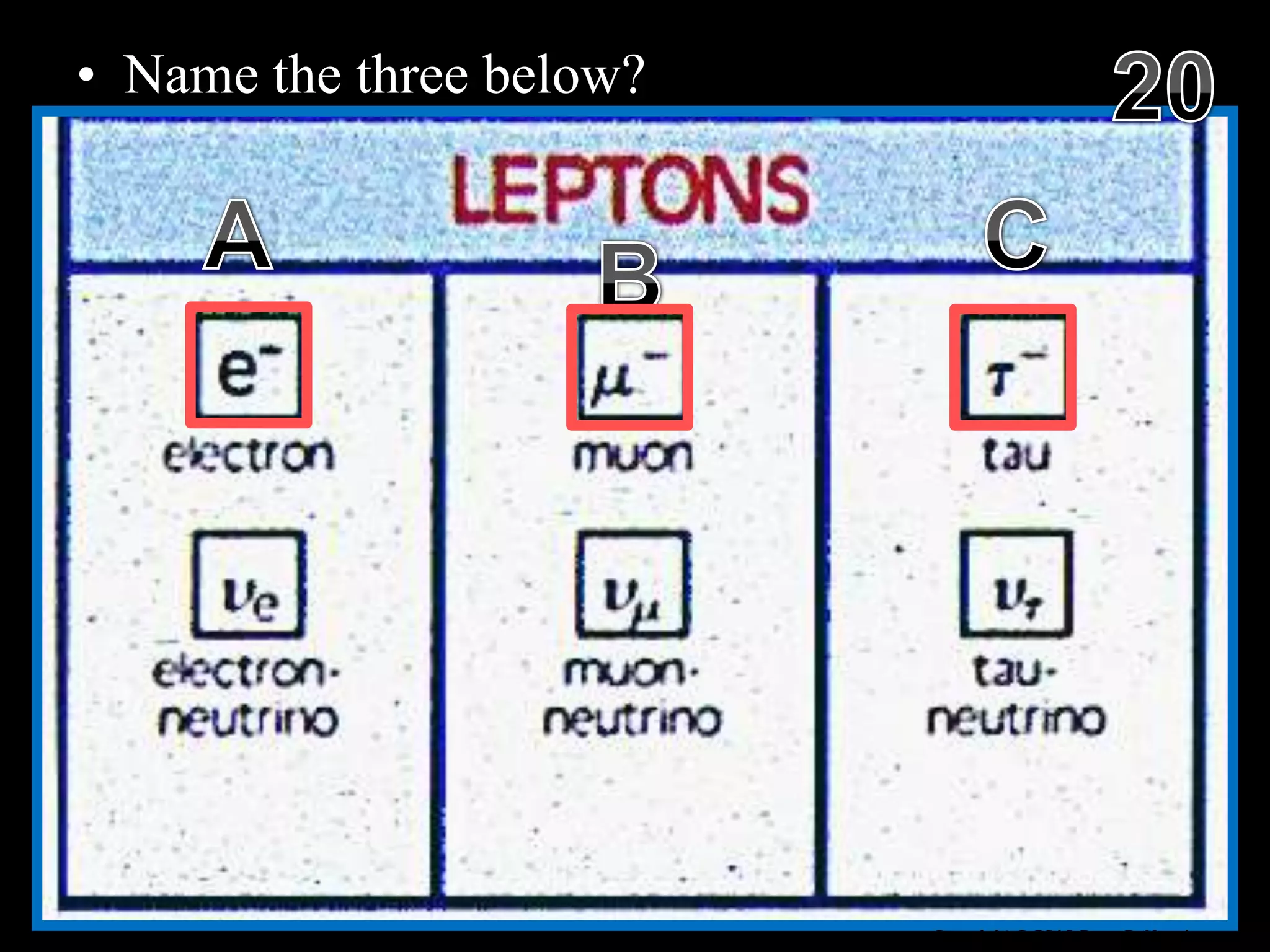
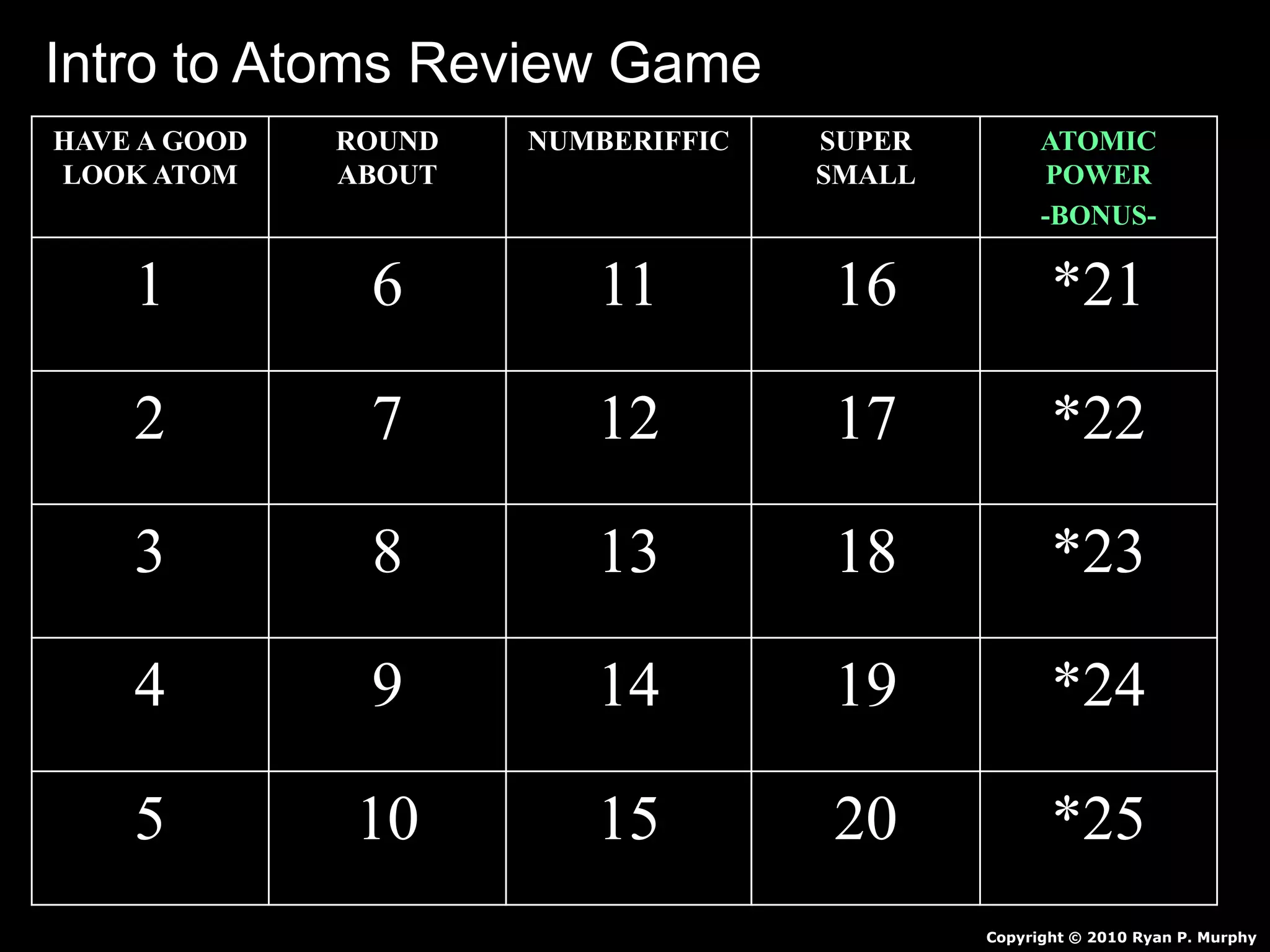
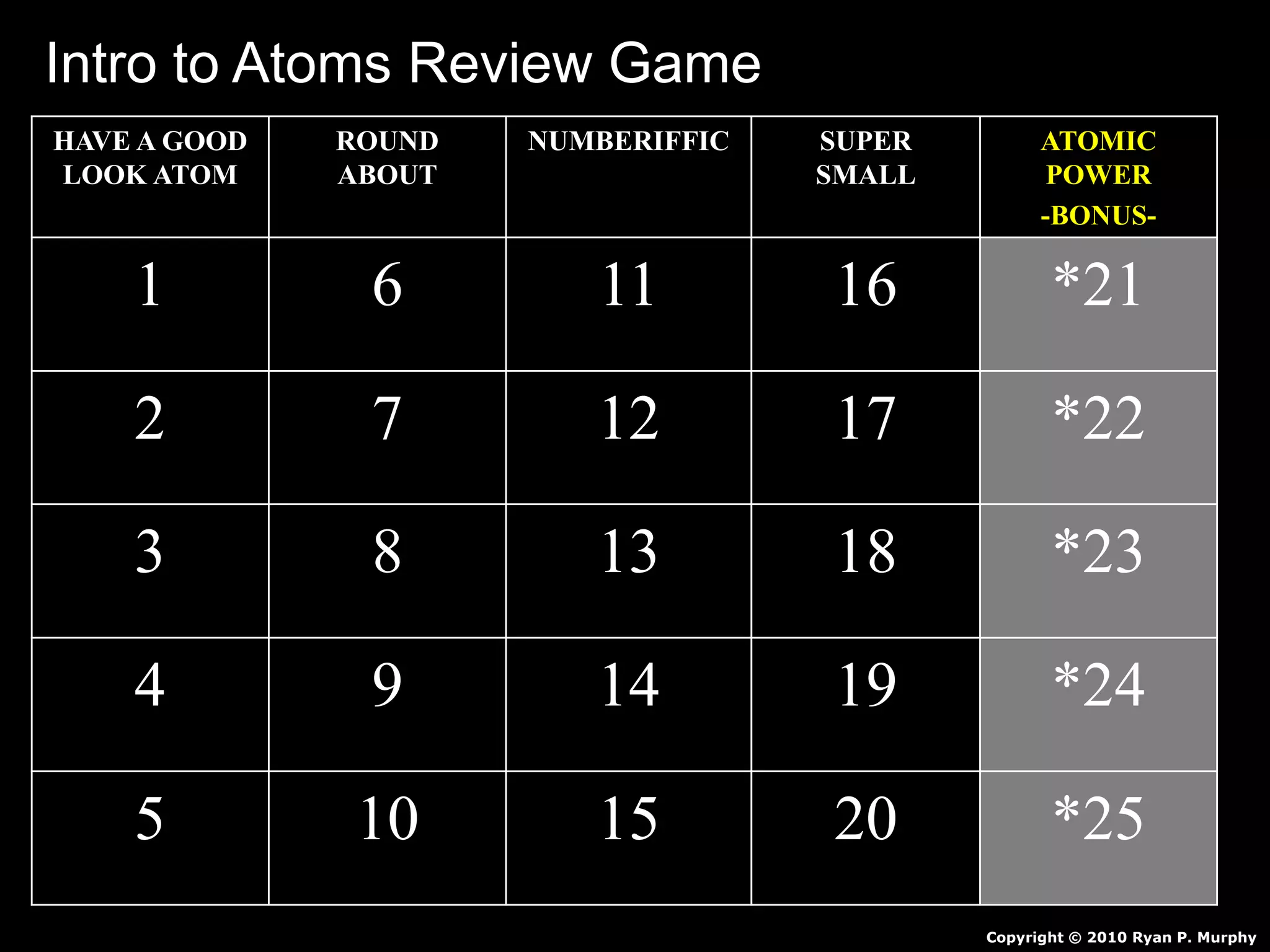
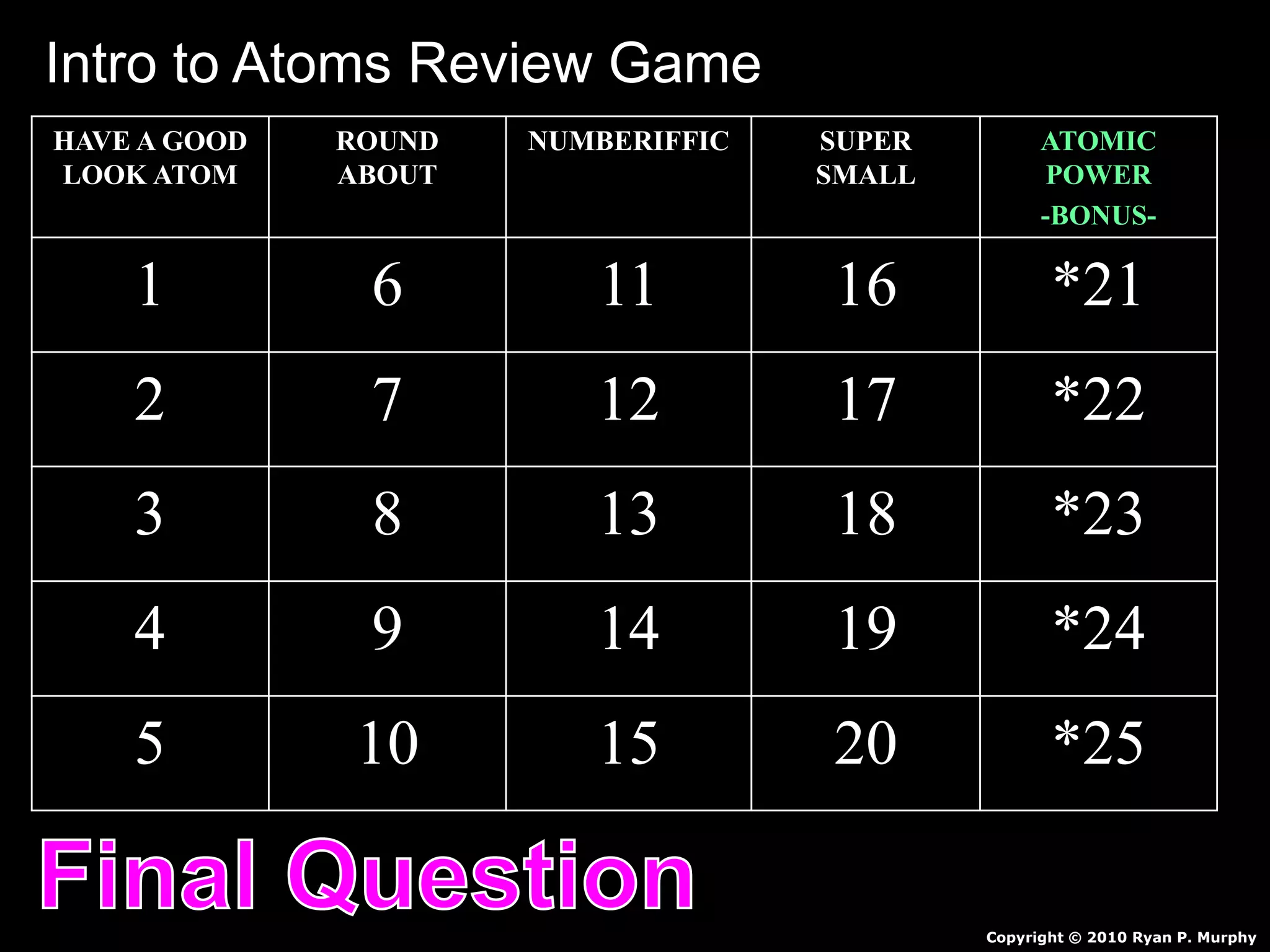
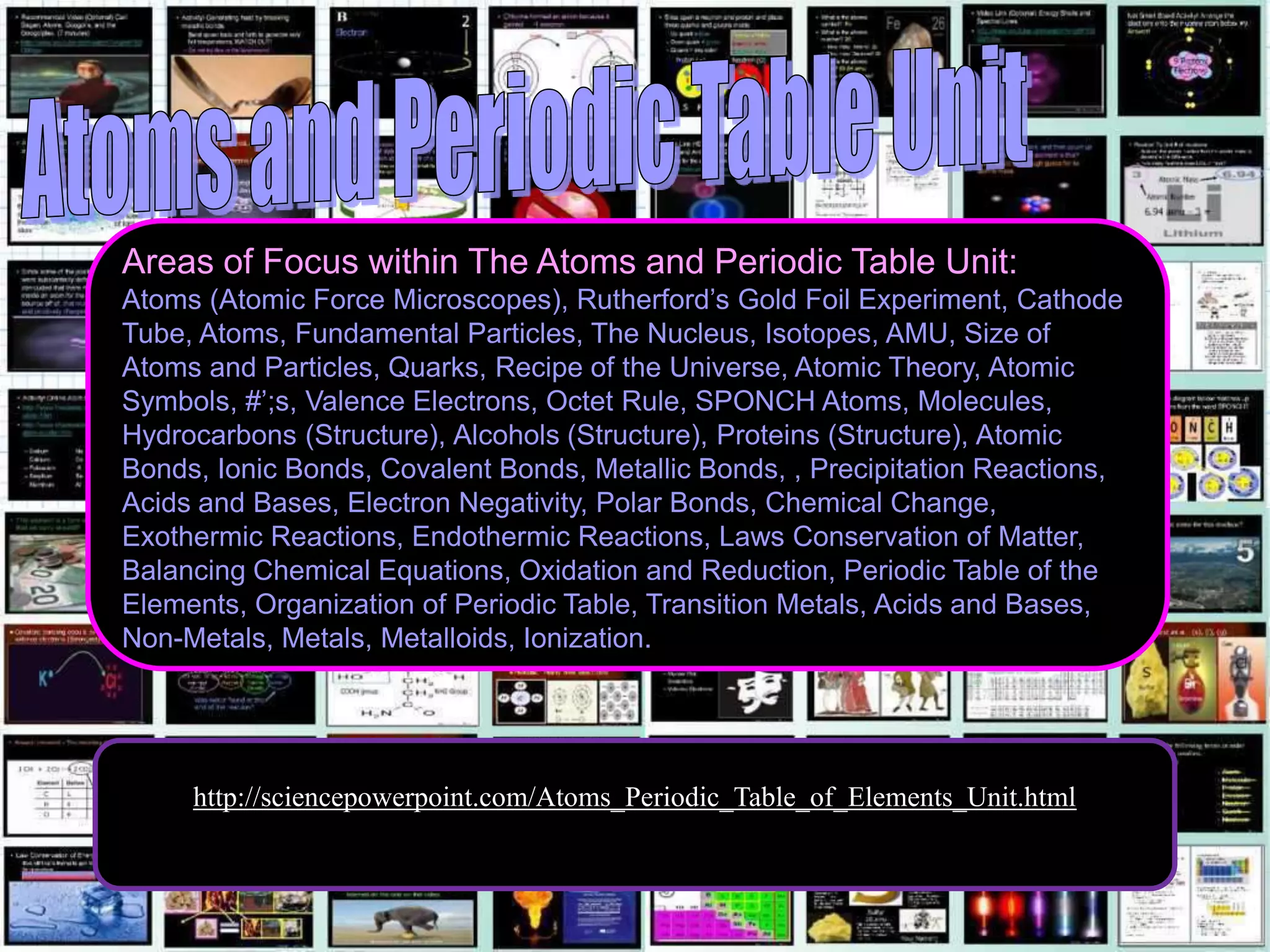
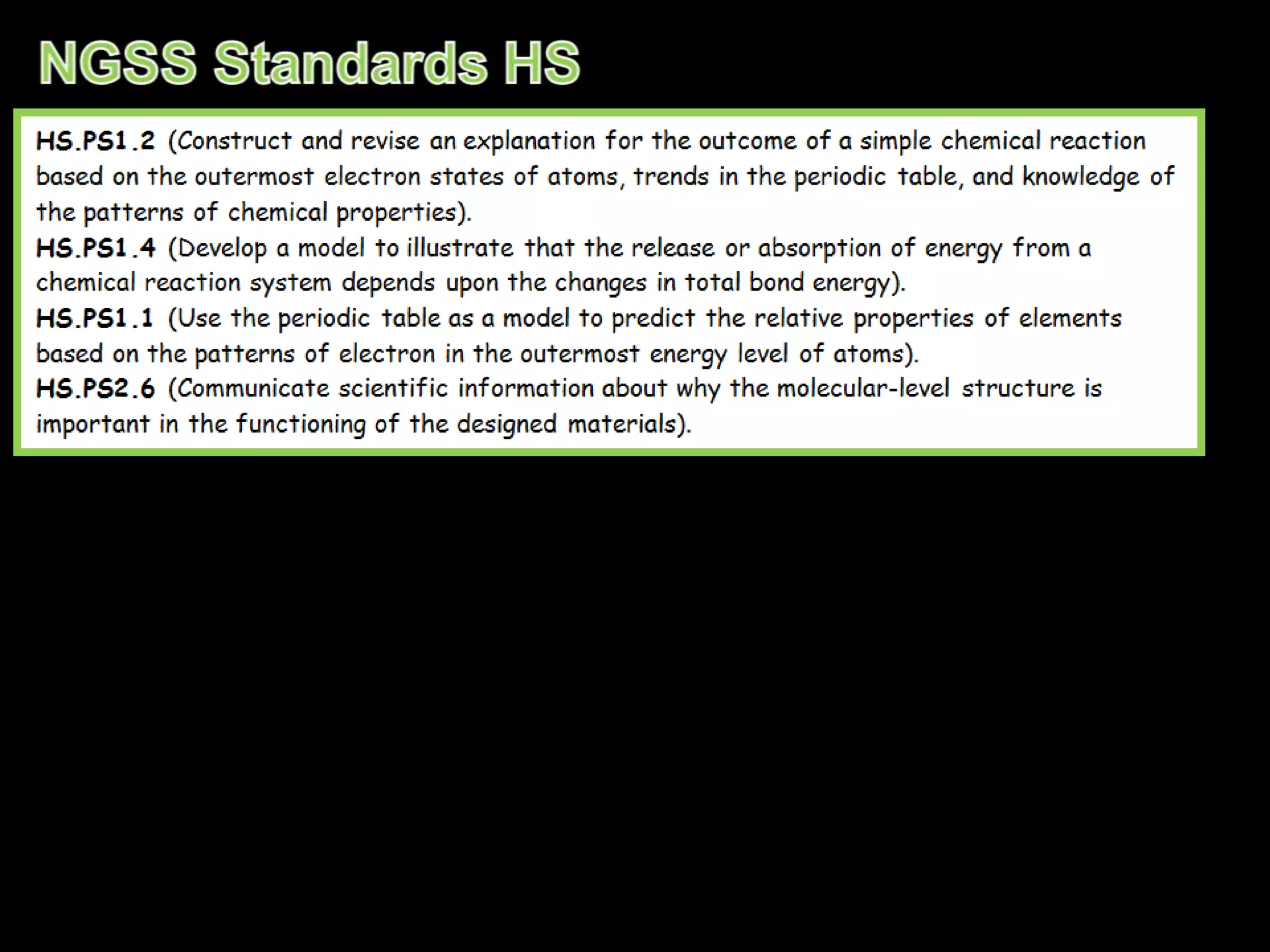
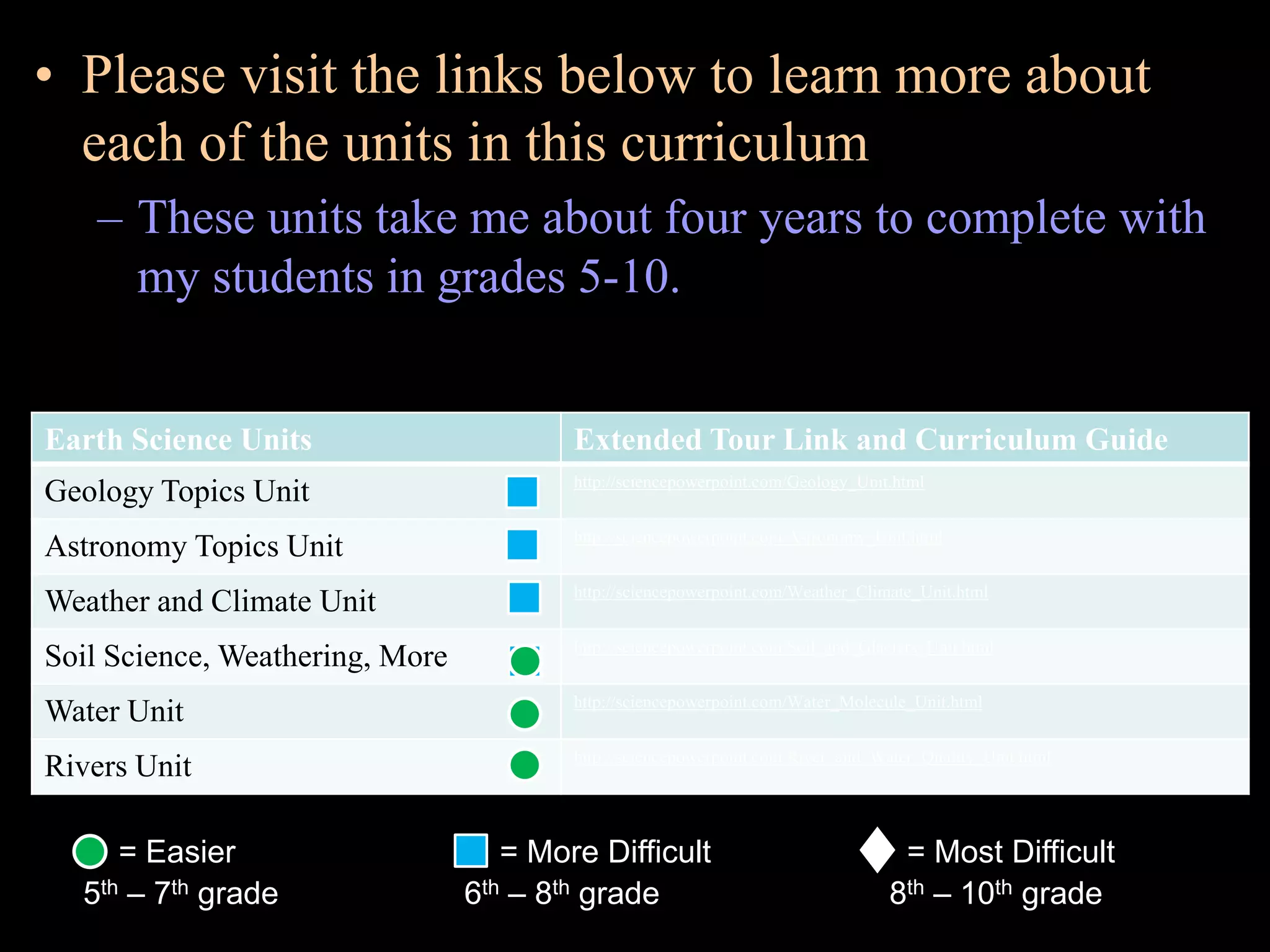
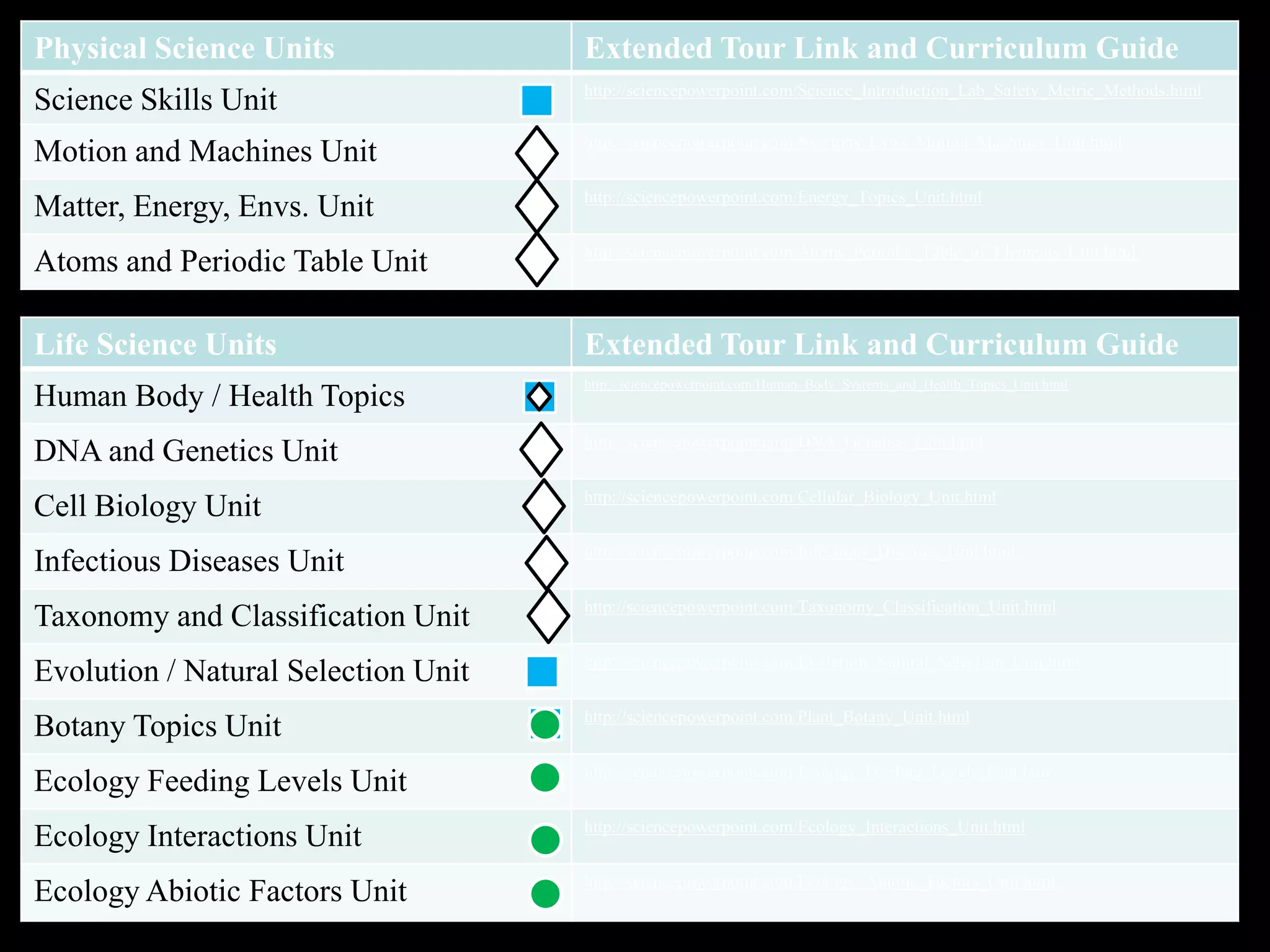
The document provides an overview of an atoms and periodic table review game for students. It includes 20 multiple choice questions about atomic structure and the periodic table, with point values assigned for each question. It also includes a bonus category at the end for additional points. The questions cover topics like atomic models, subatomic particles, isotopes, and elements on the periodic table. The review game is intended to help students assess their understanding of key concepts from the atoms and periodic table unit.