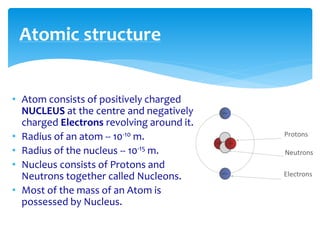
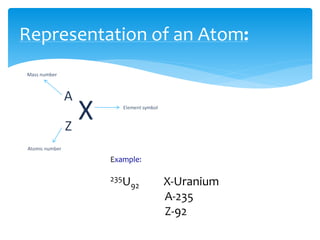
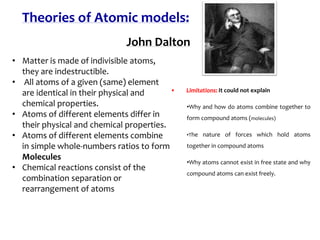
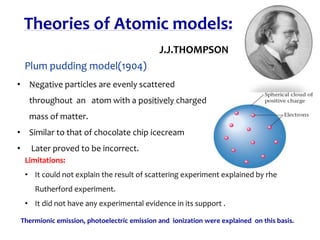
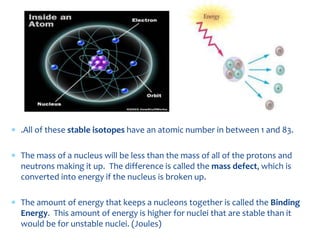
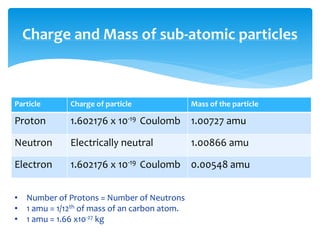
1. Atoms are the basic building blocks of matter and consist of a small, dense nucleus surrounded by electrons.
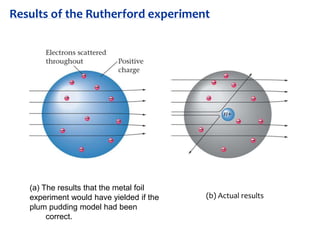
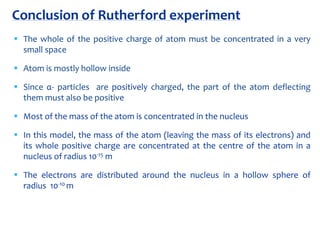
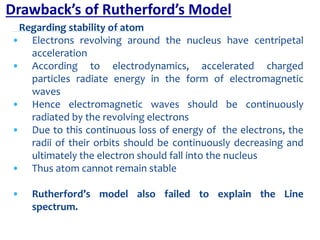
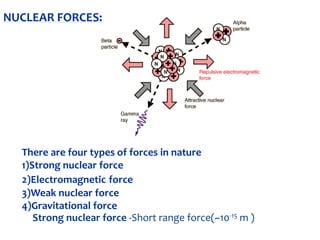
2. Rutherford's gold foil experiment in 1911 showed that the atom has a small, dense nucleus containing positively charged protons and uncharged neutrons.
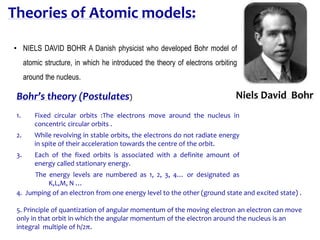
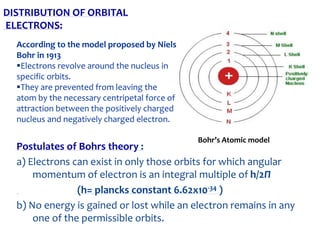
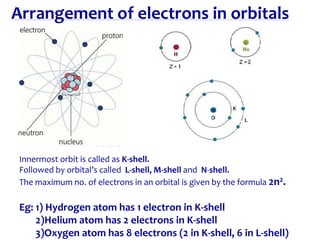
3. Niels Bohr proposed his model of the atom in 1913 in which electrons orbit the nucleus in fixed shells at specific energy levels, explaining atomic spectra.