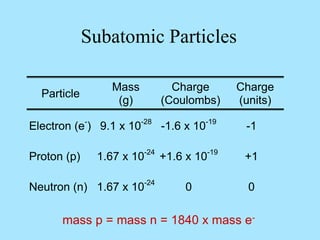
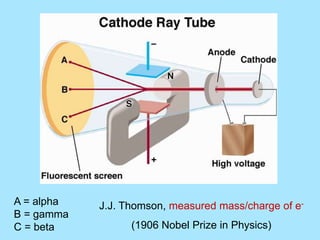
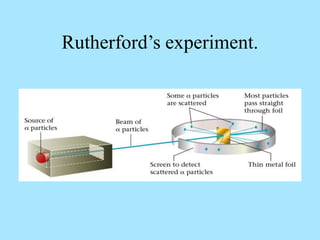
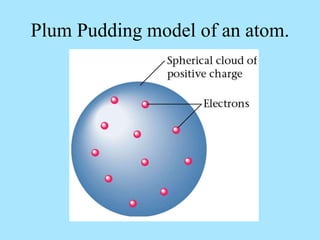
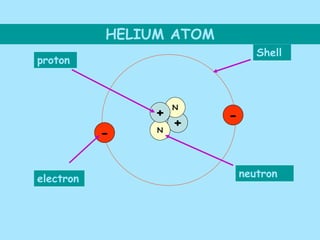
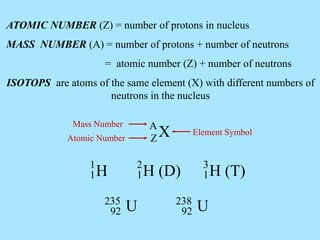
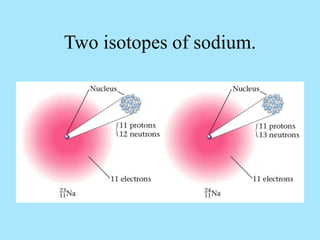
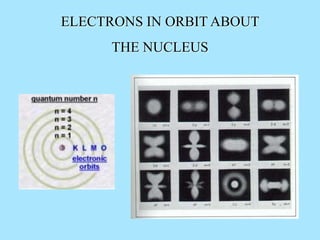
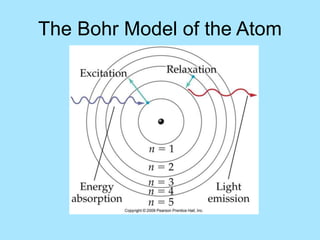
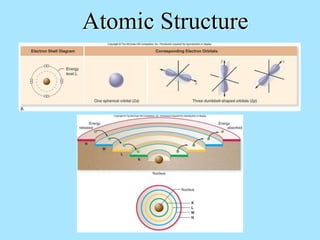
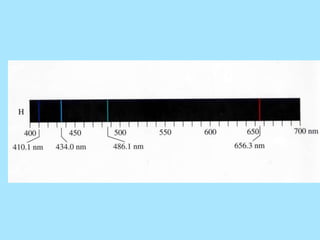
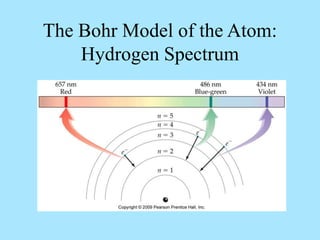
Atoms are composed of protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, while electrons surround the nucleus in orbitals. Over time, scientists such as Dalton, Thomson, Rutherford, and Bohr contributed to the developing atomic model. Bohr refined Rutherford's model by proposing that electrons exist in specific energy levels or orbits around the nucleus. Elements have unique atomic structures that are indicated by their atomic number and mass number.