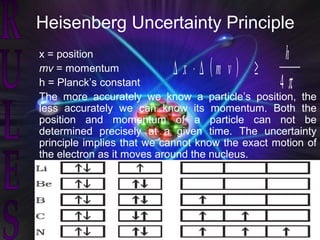
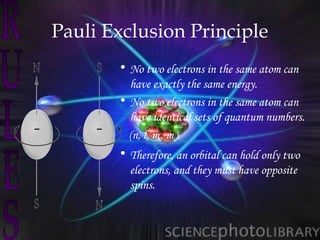
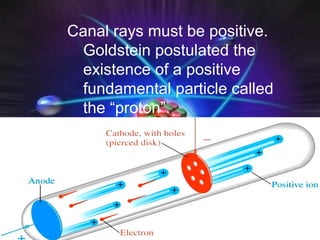
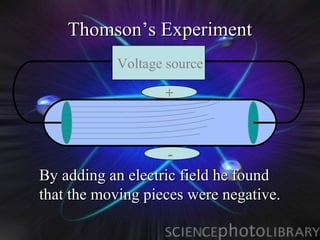
Atomic structure and models have evolved over time through discoveries and experiments:
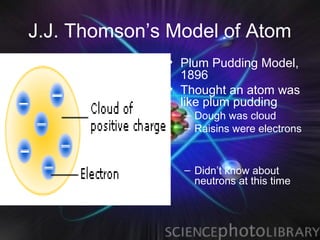
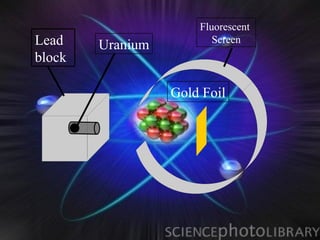
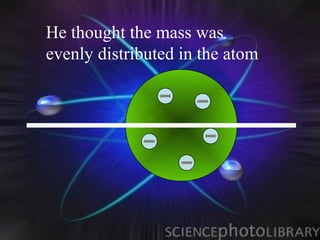
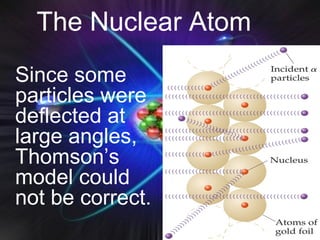
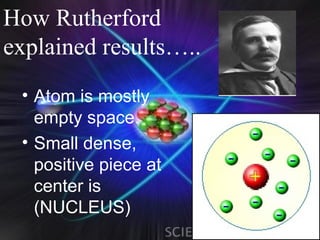
(1) Rutherford discovered the nucleus through deflection of alpha particles, replacing Thomson's plum pudding model.
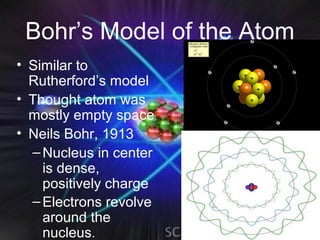
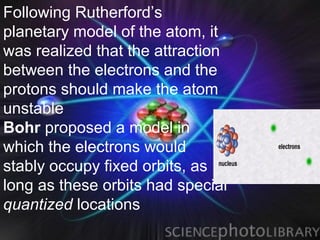
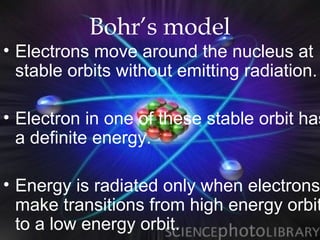
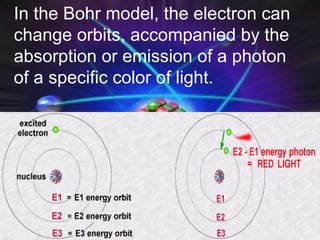
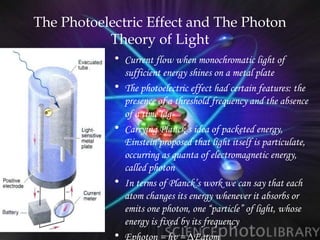
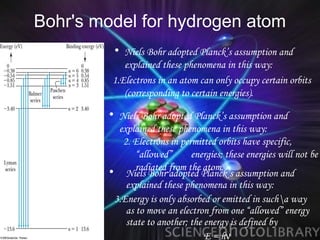
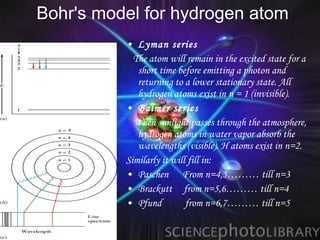
(2) Bohr incorporated Planck's quantization of energy to explain the stability of atoms in his planetary model of electrons in fixed orbits around the nucleus.
(3) Later models such as Heisenberg's uncertainty principle and the Pauli exclusion principle further refined the quantum mechanical model of electrons and orbitals in atoms.






















![Hund’s Rule
• The lowest energy
configuration for an atom is the
one having the maximum
number of unpaired electrons
allowed by the Pauli principle in
a particular set of degenerate
orbitals.
• N : 1s2 2s2 2p3, O : 1s2 2s2
2p4,
• F : 1s2 2s2 2p5, Ne : 1s2
2s2 2p6,
• Na : 1s2 2s2 2p63s1 OR
[Ne] 3s1](https://image.slidesharecdn.com/structurofatom-130412223536-phpapp01/85/Structur-of-atom-by-alex-42-320.jpg)