This document provides an overview of key concepts related to the chemical basis of life, including:
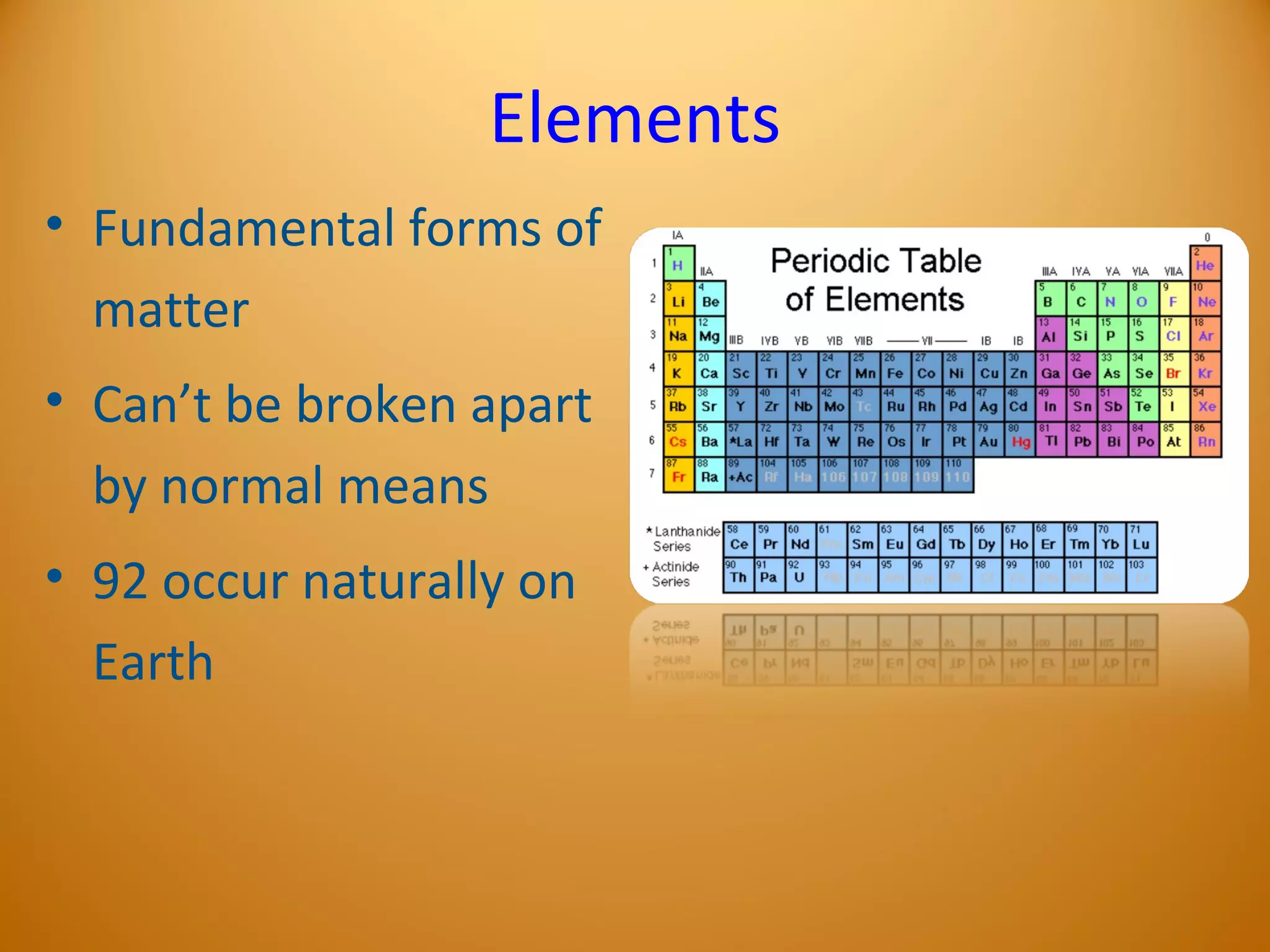
1) Elements are the fundamental forms of matter that cannot be broken down further through normal means. The most common elements in the human body are oxygen, hydrogen, carbon, nitrogen, and calcium.
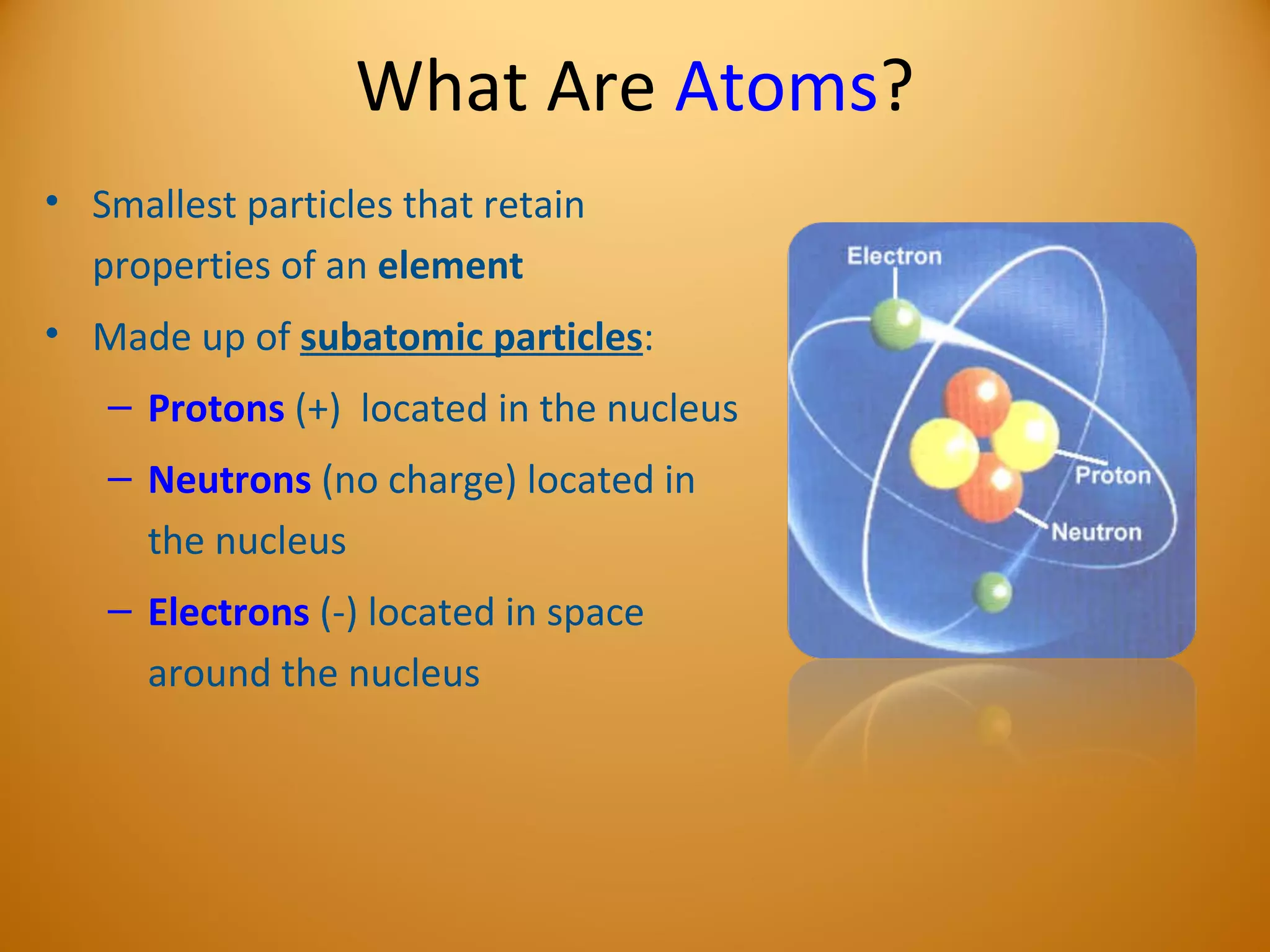
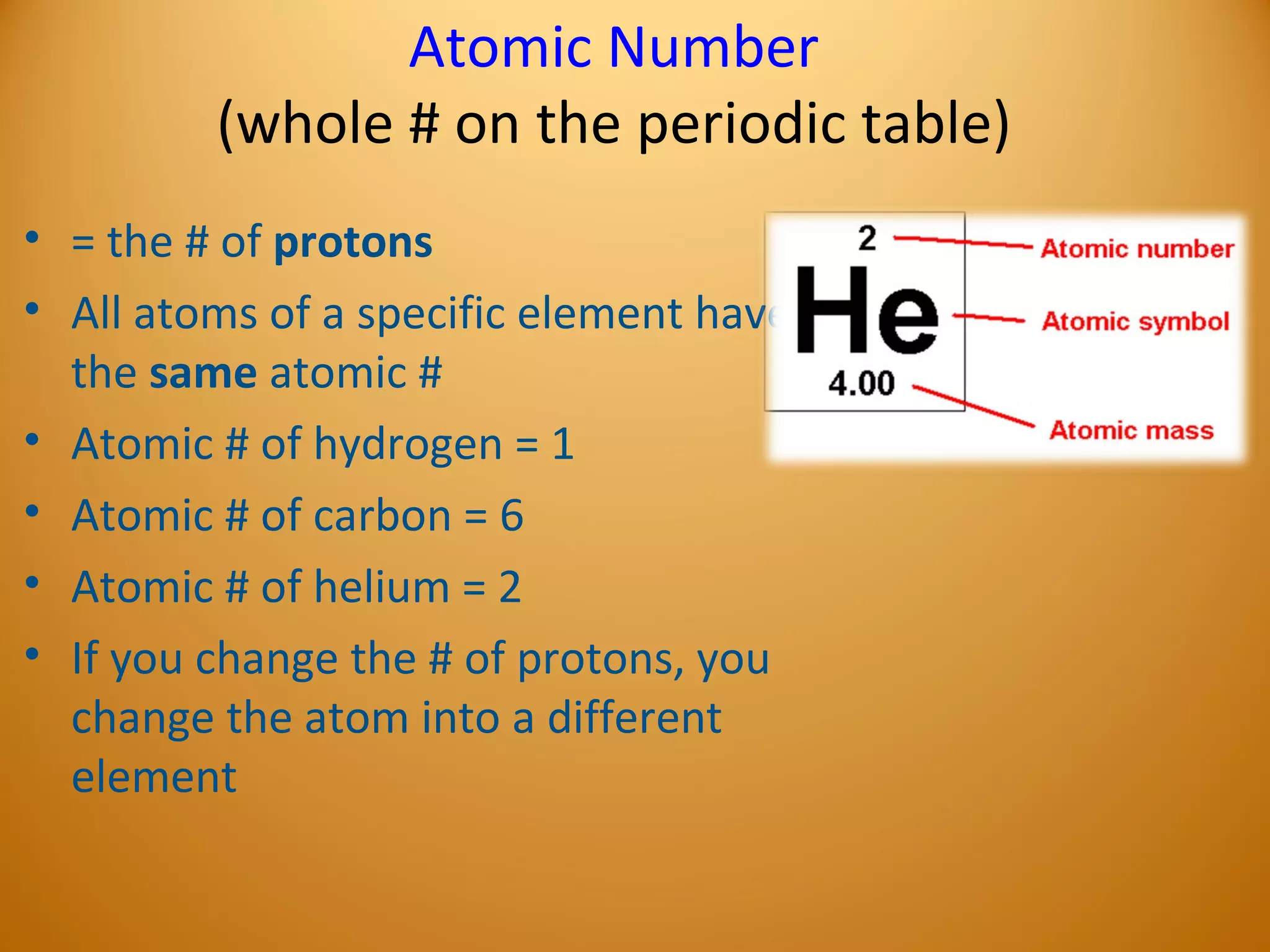
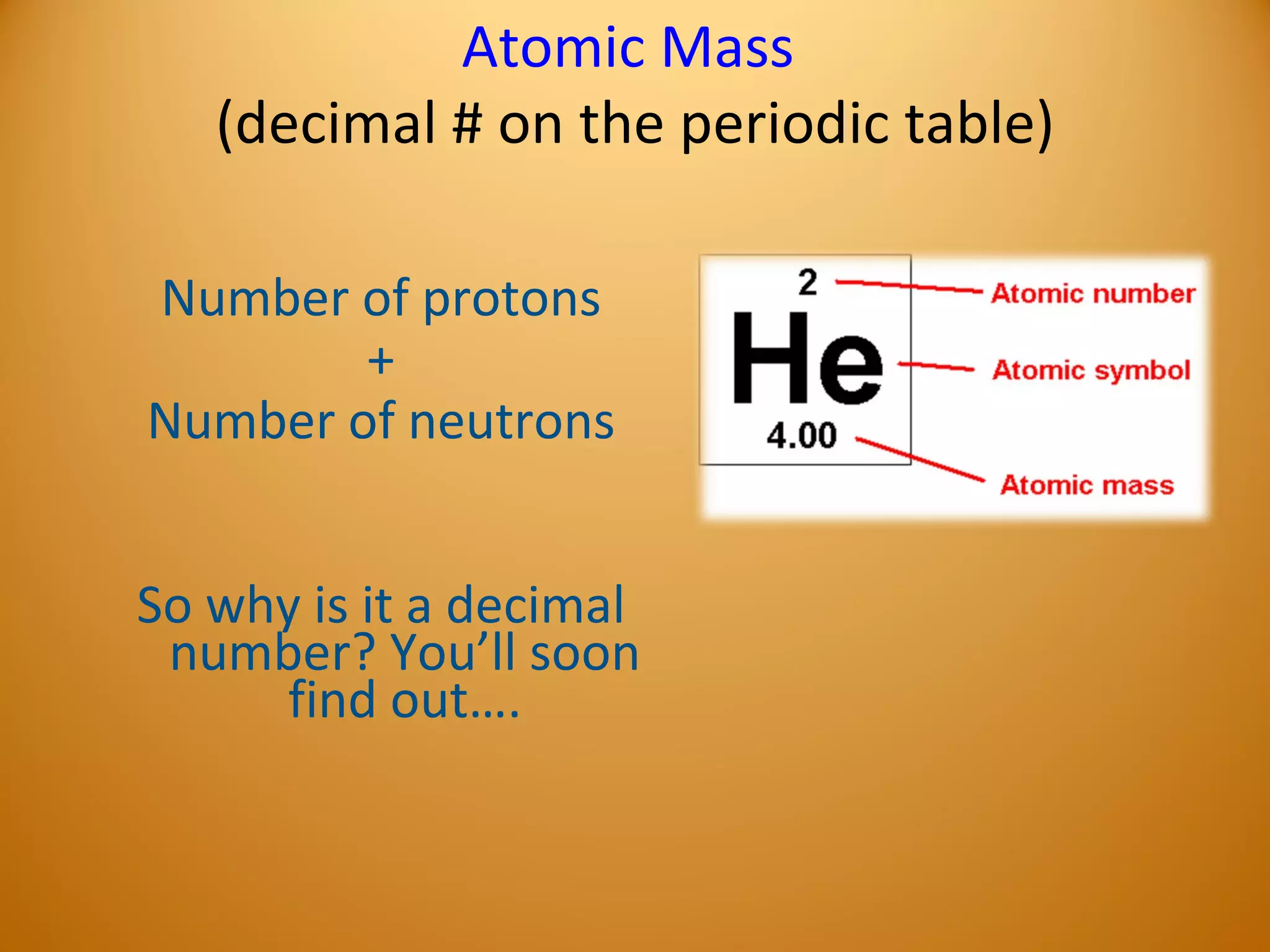
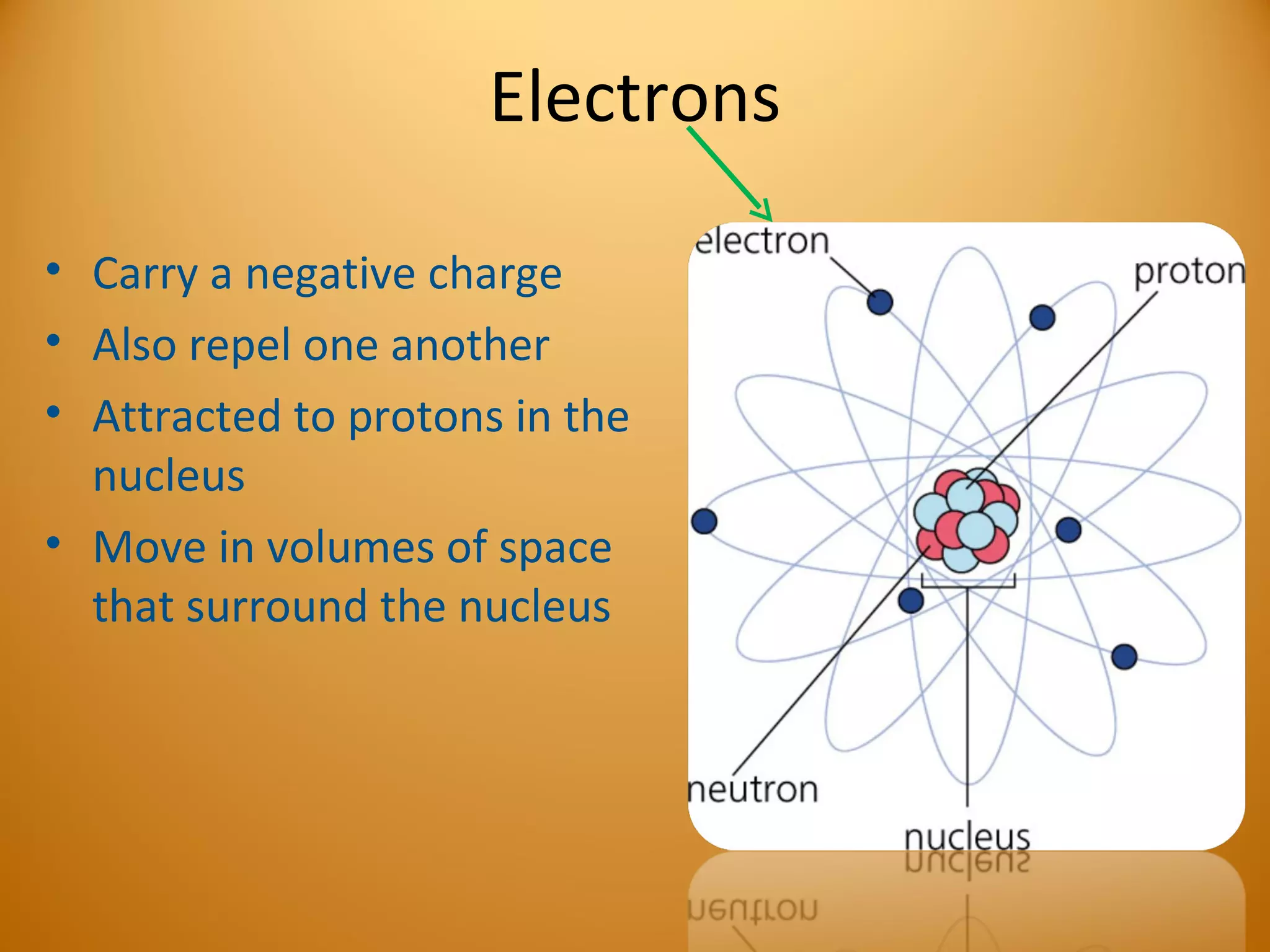
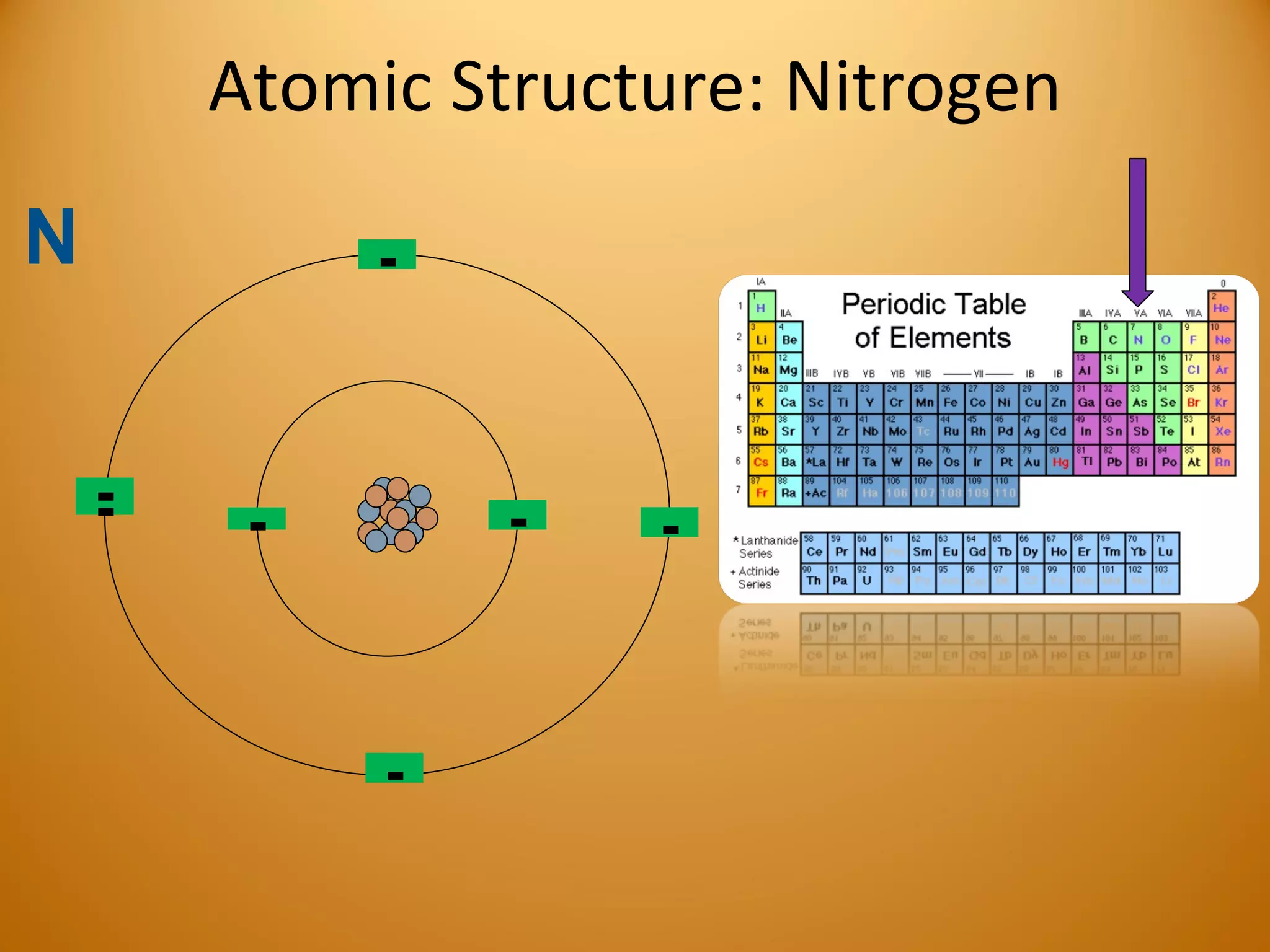
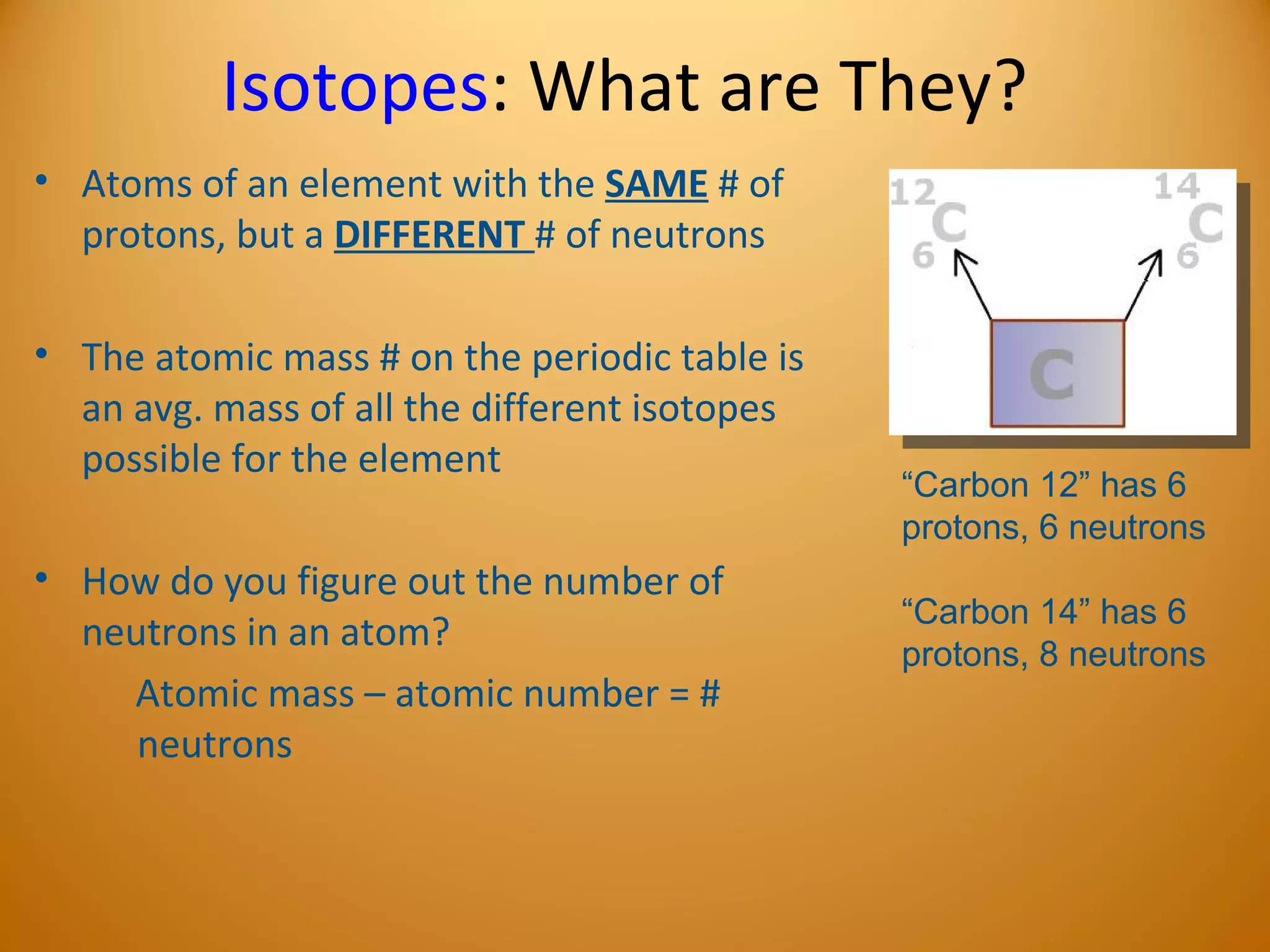
2) Atoms are made up of subatomic particles including protons, neutrons, and electrons. The number of protons determines the element, while the number of neutrons can vary between isotopes of the same element.
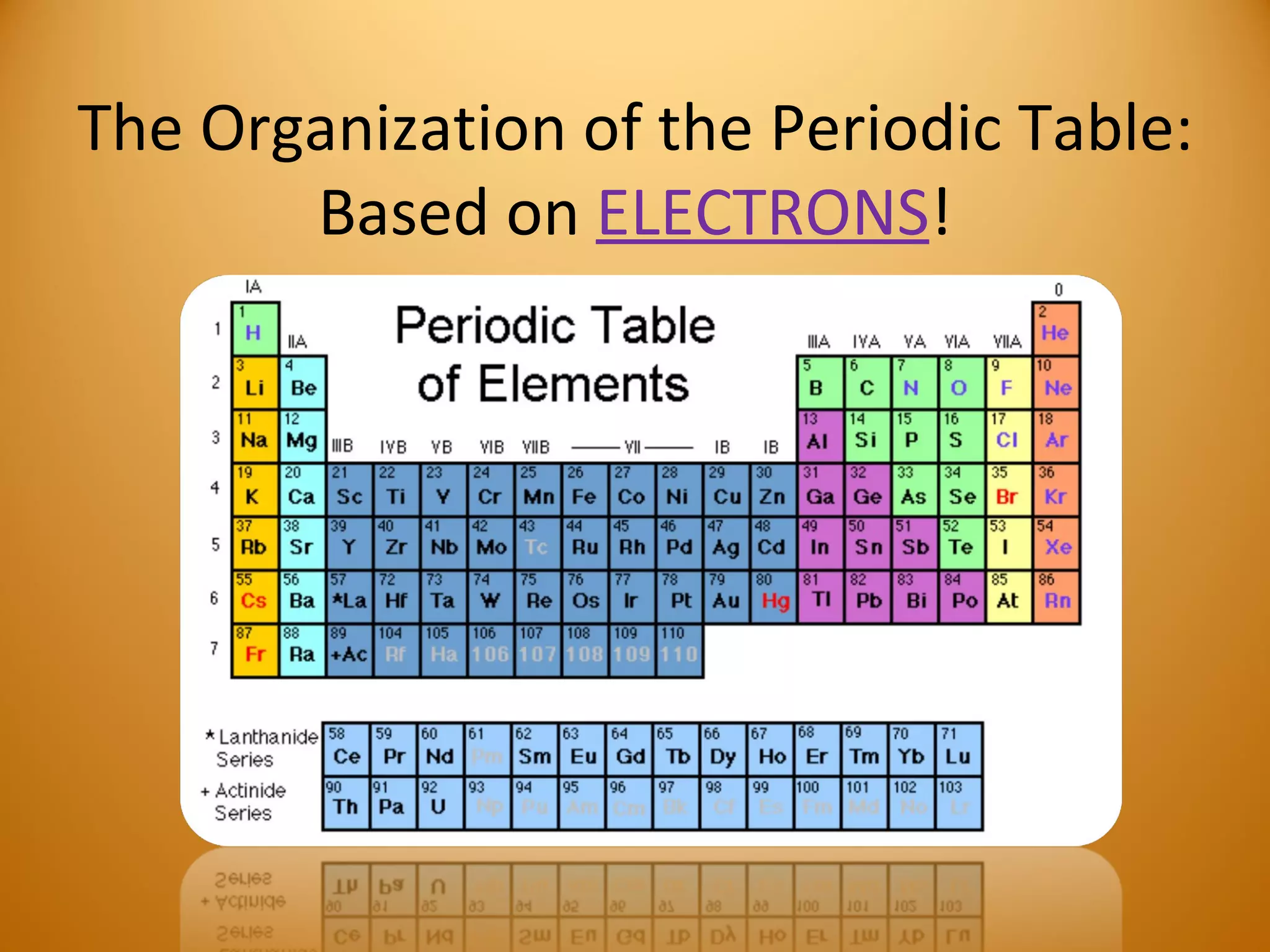
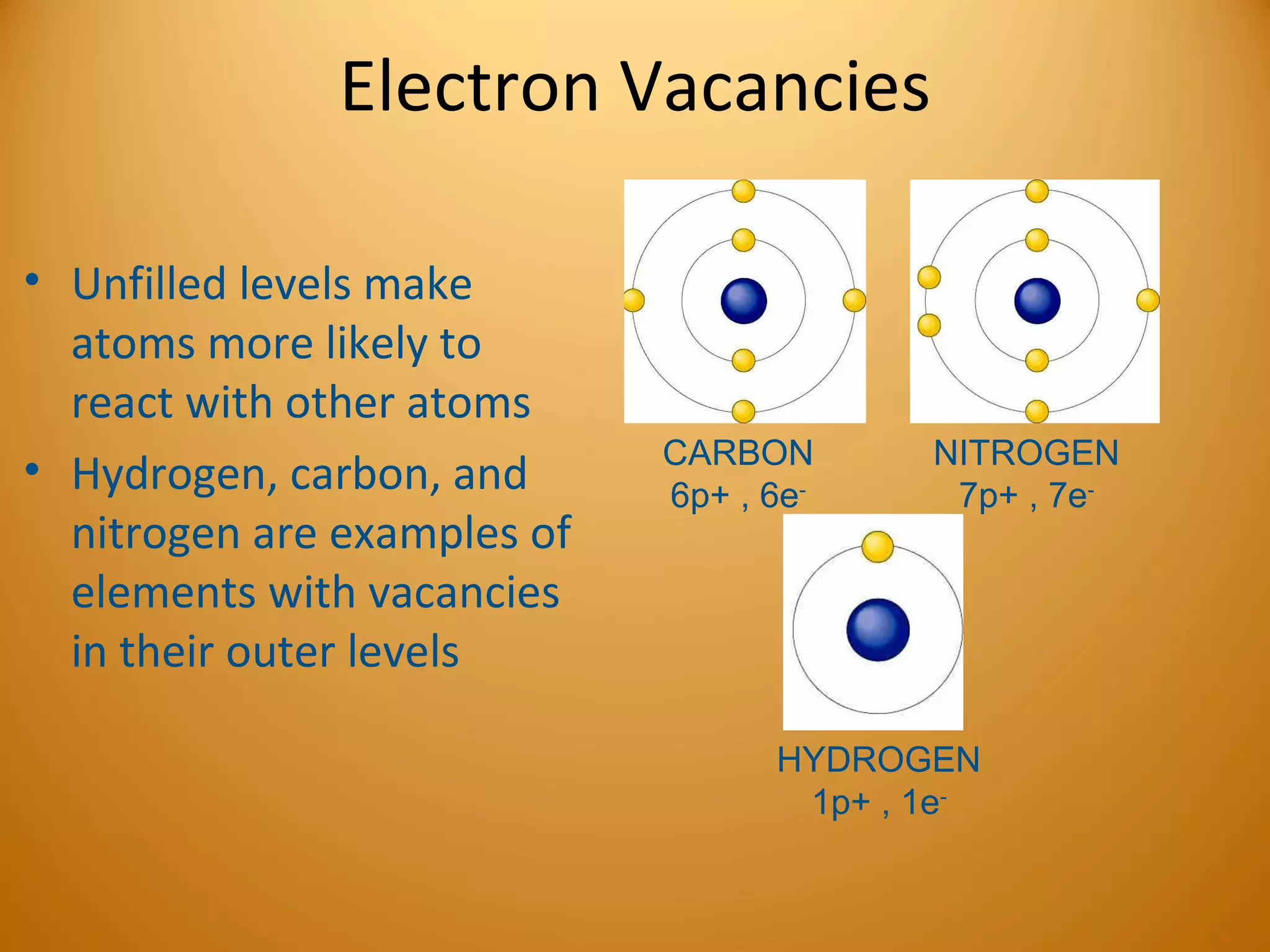
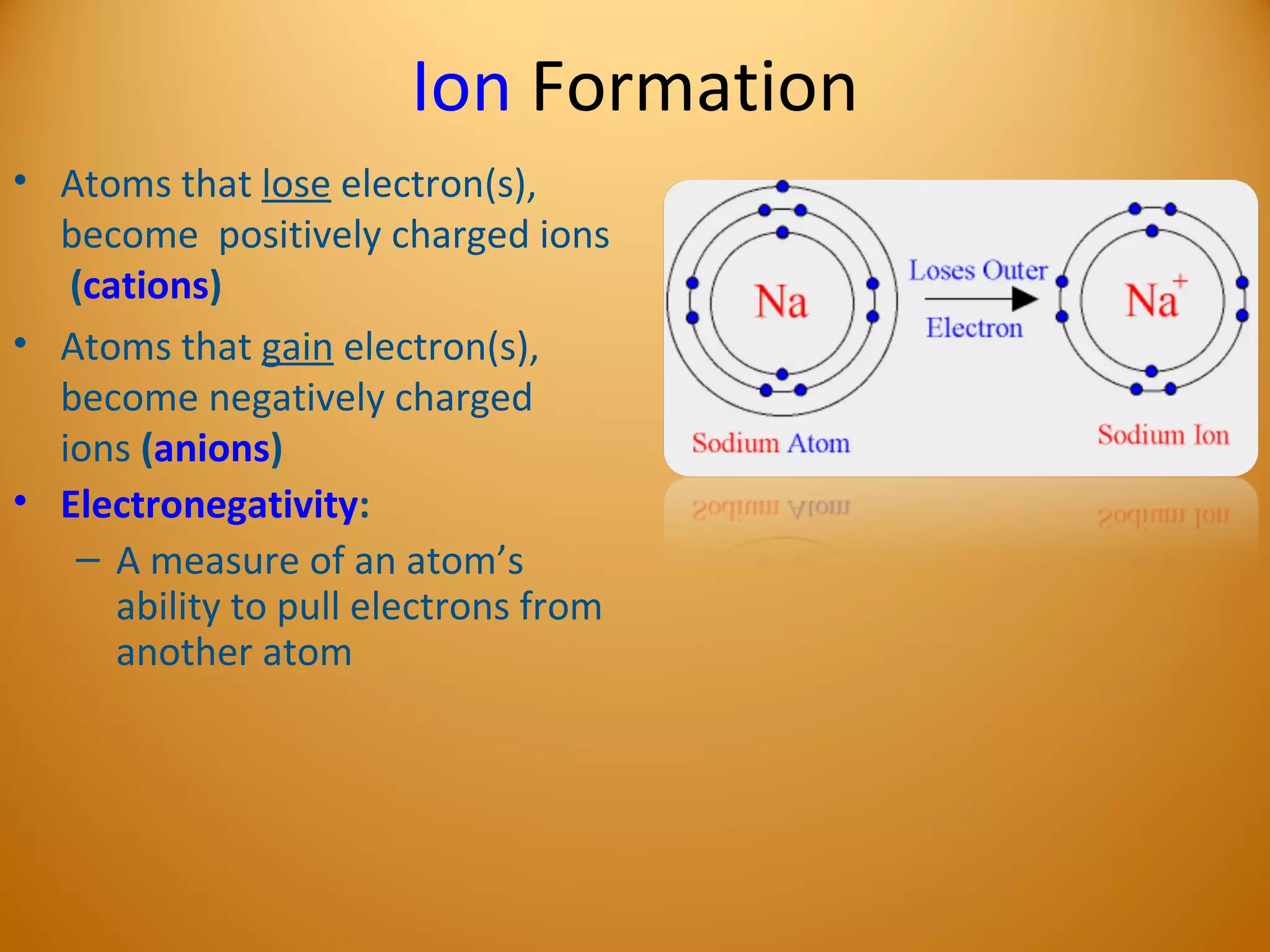
3) Electrons play an important role in how atoms interact, forming ions by gaining or losing electrons or bonding through electron sharing. The organization of the periodic table is based on electron configurations.