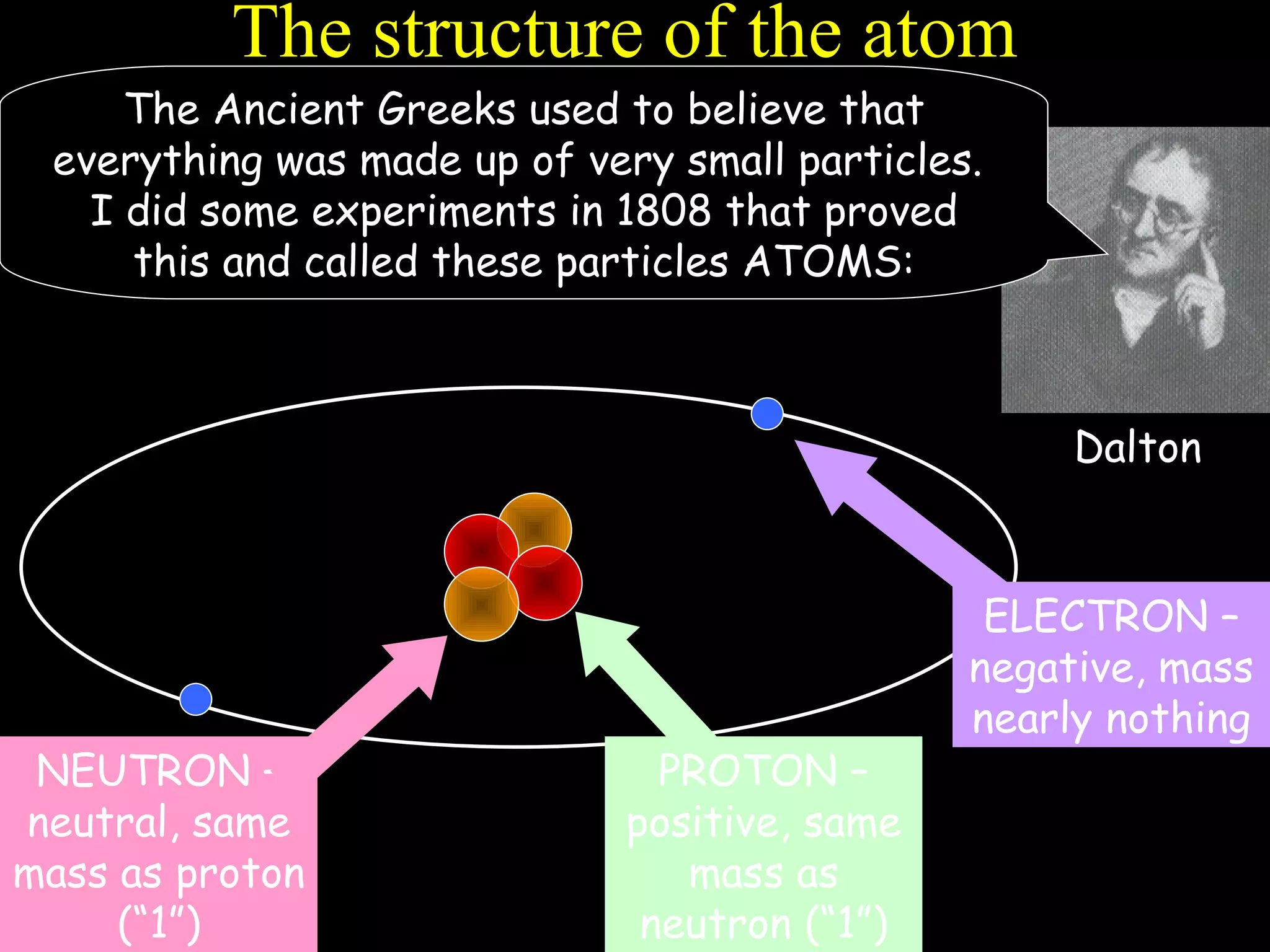
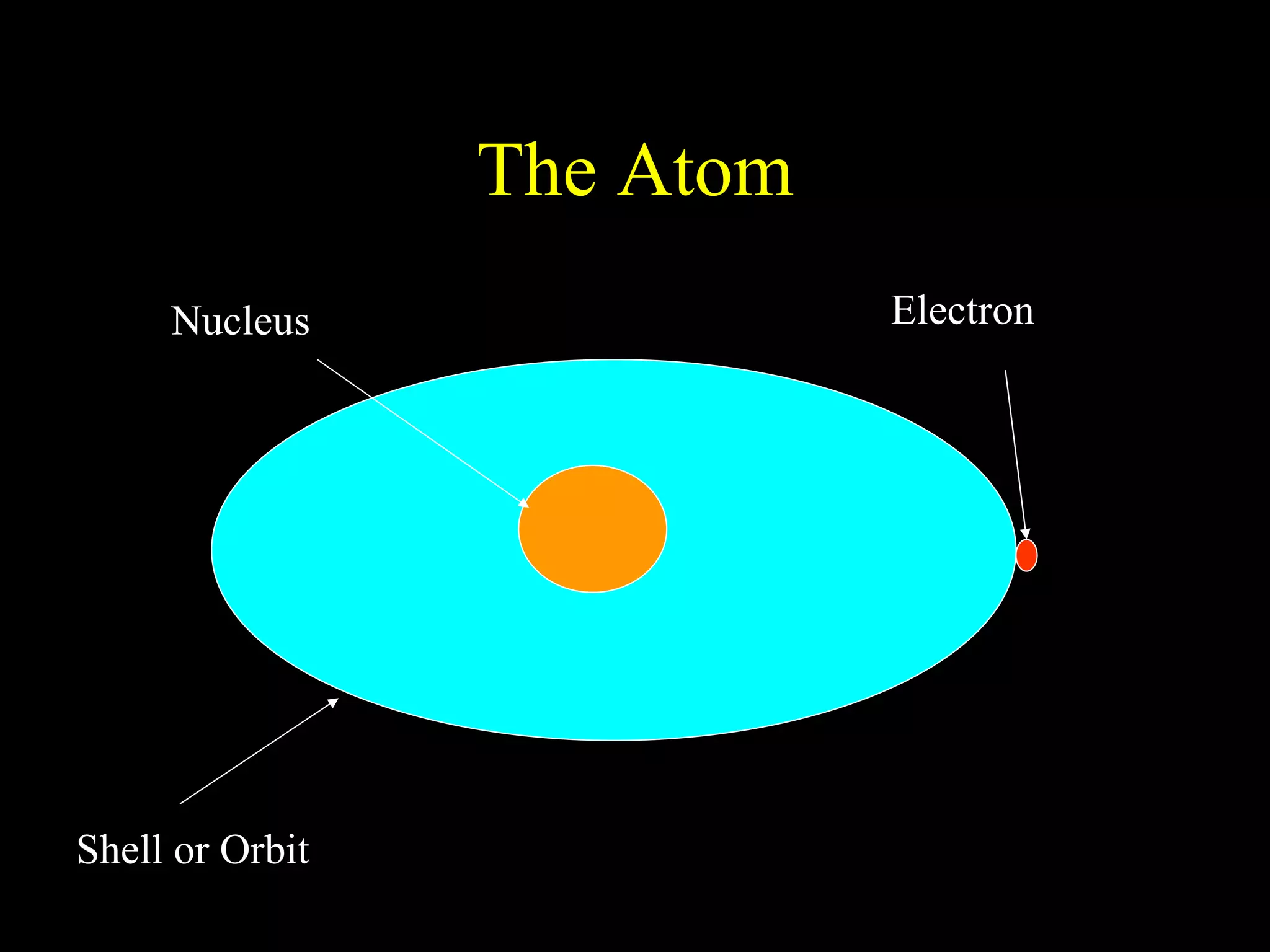
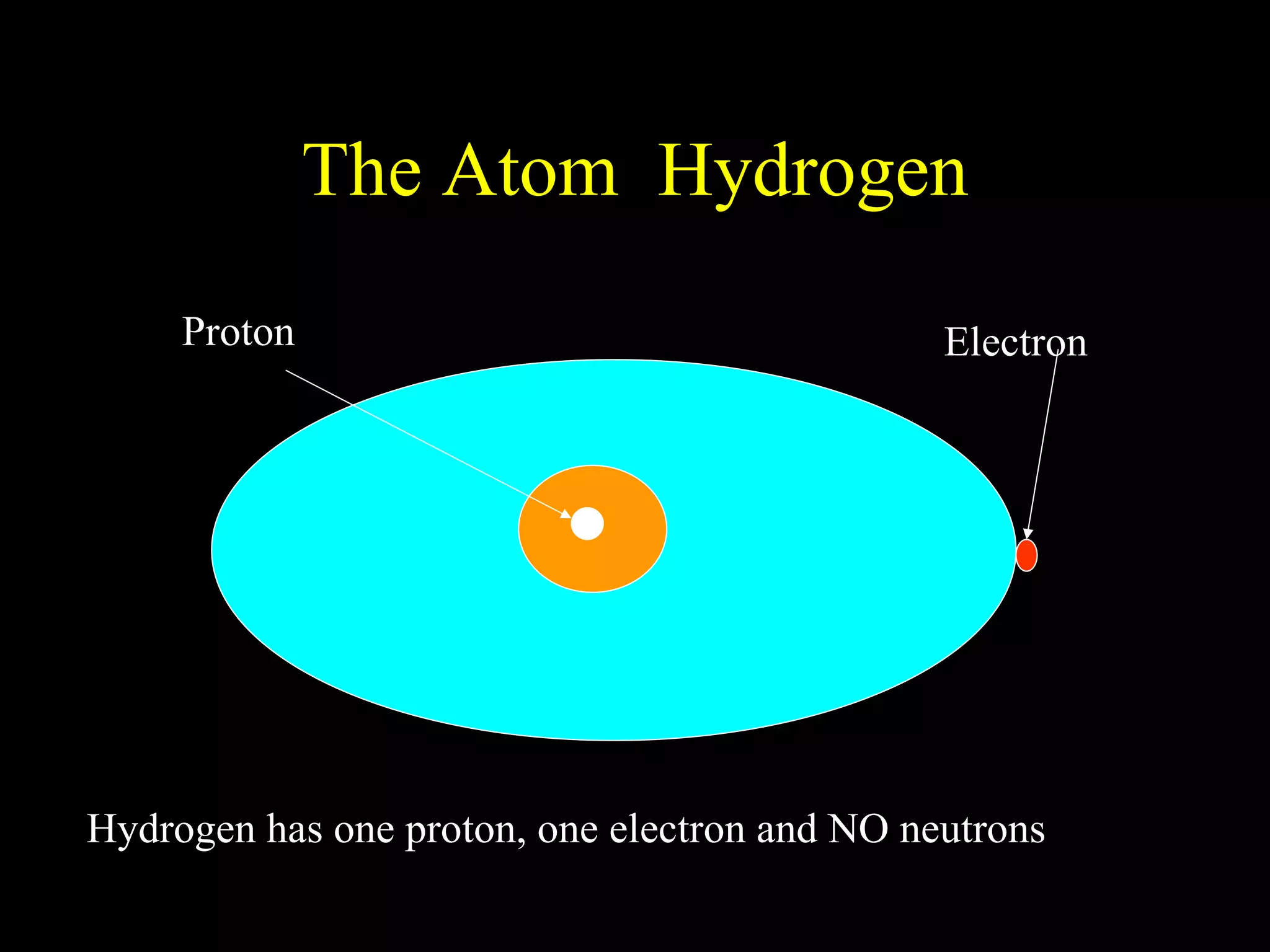
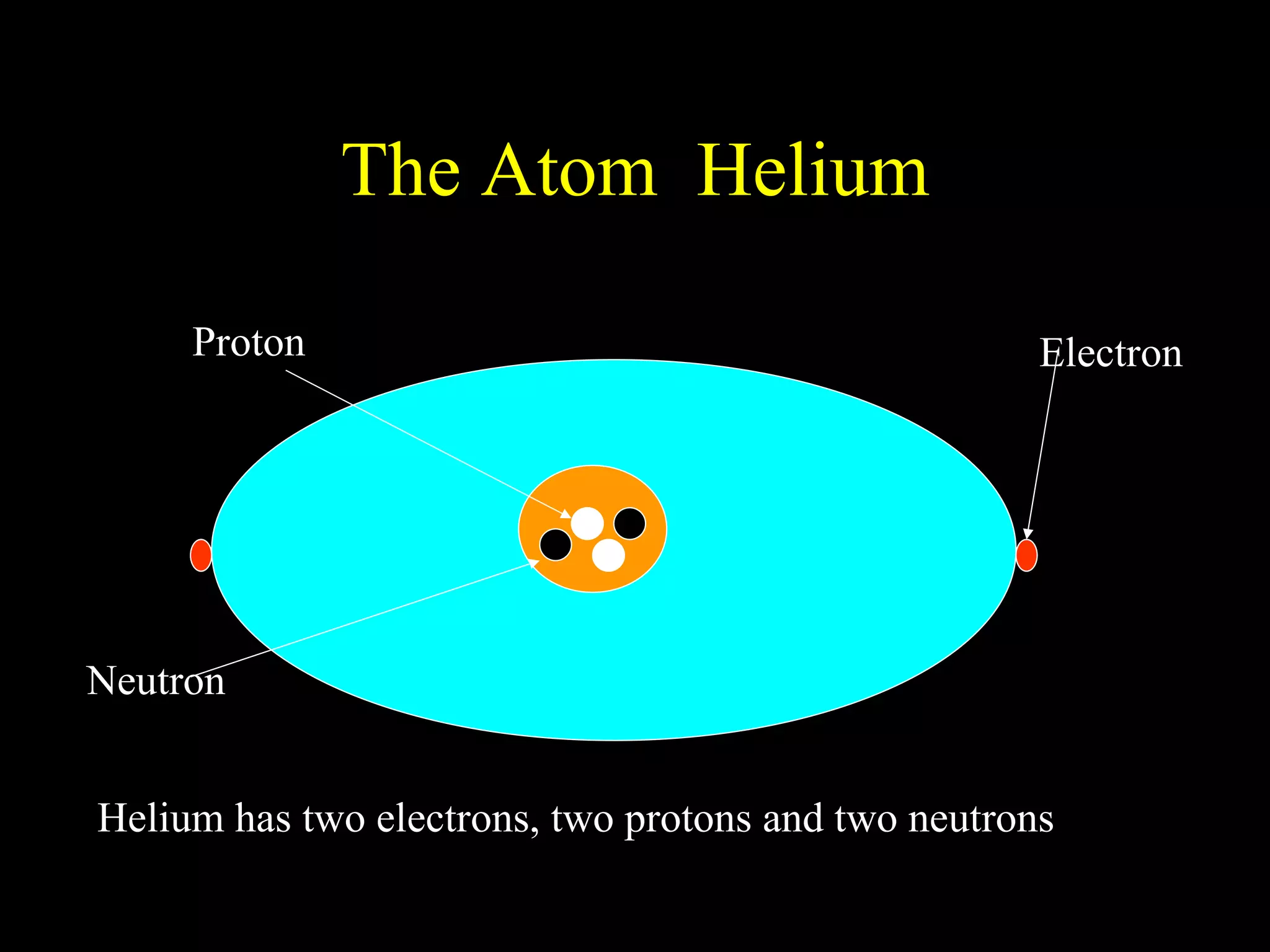
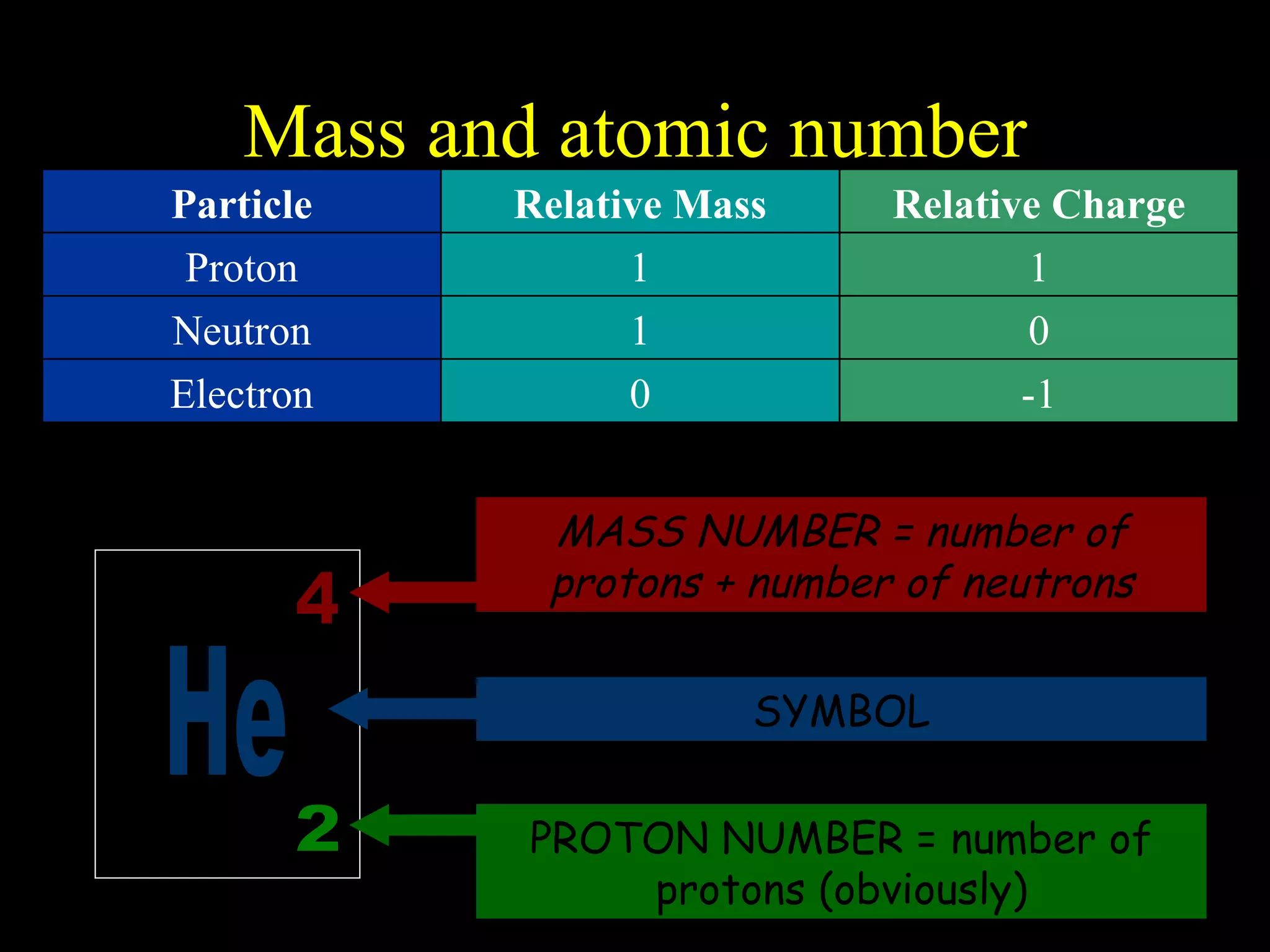
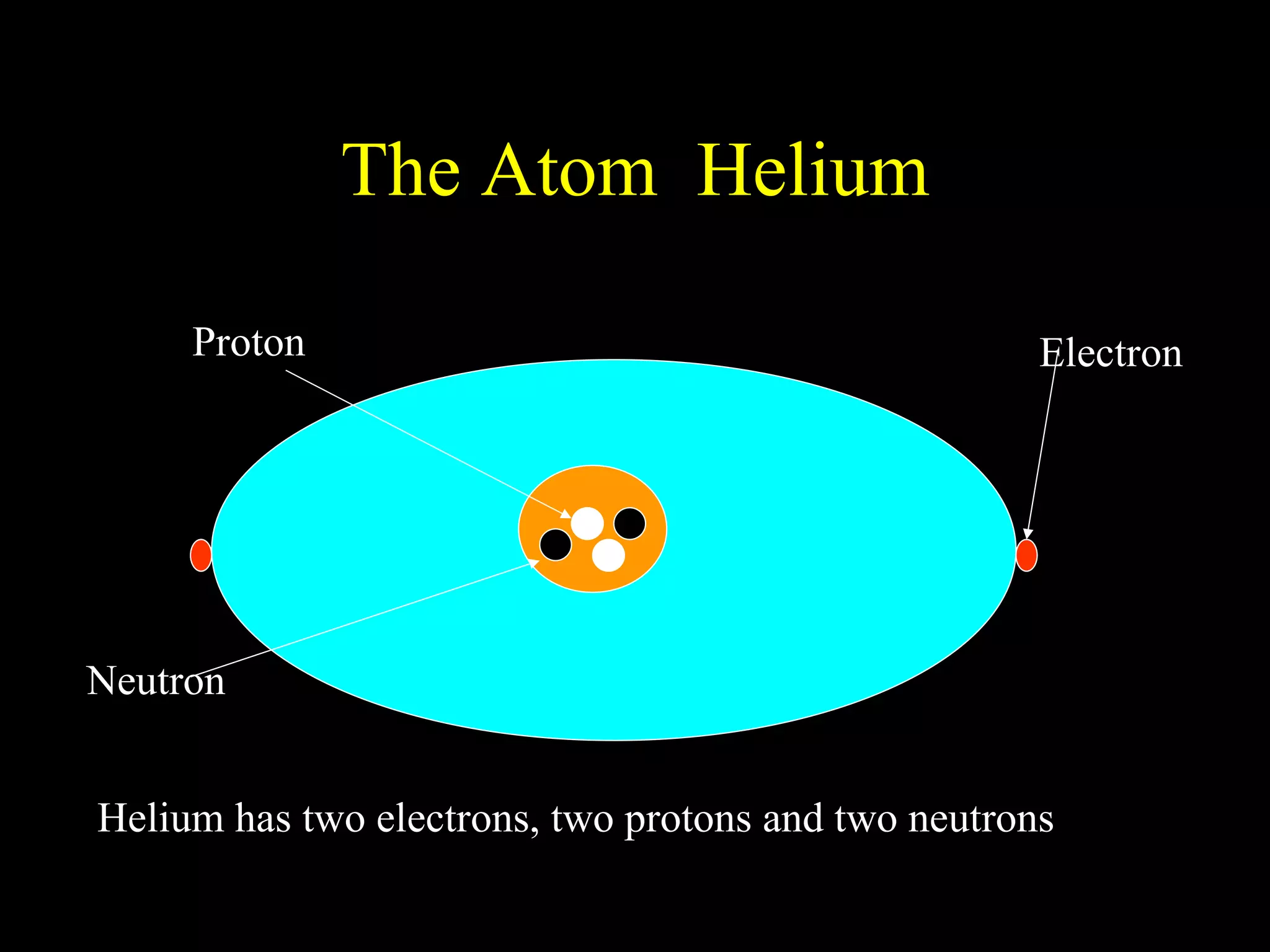
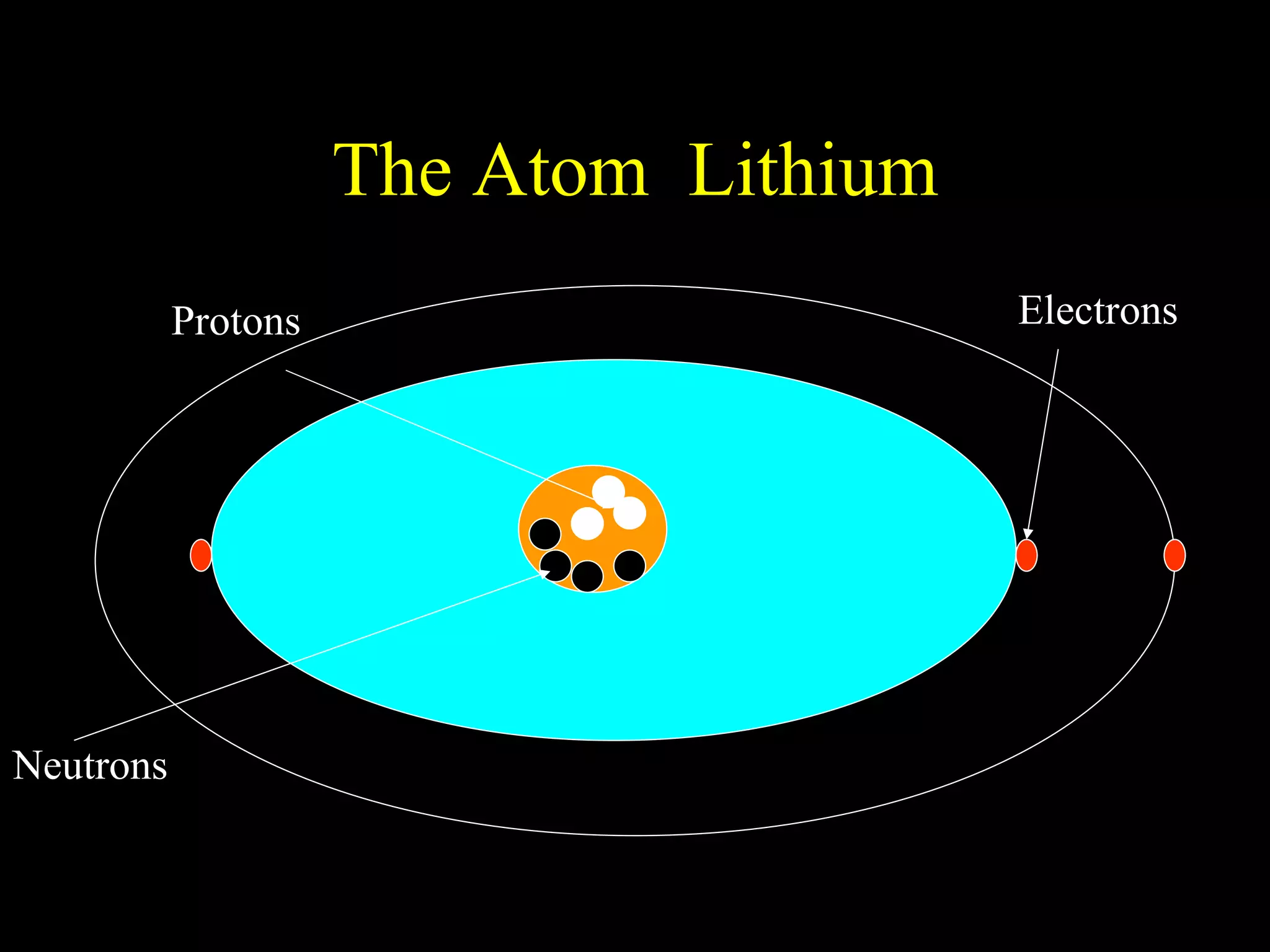
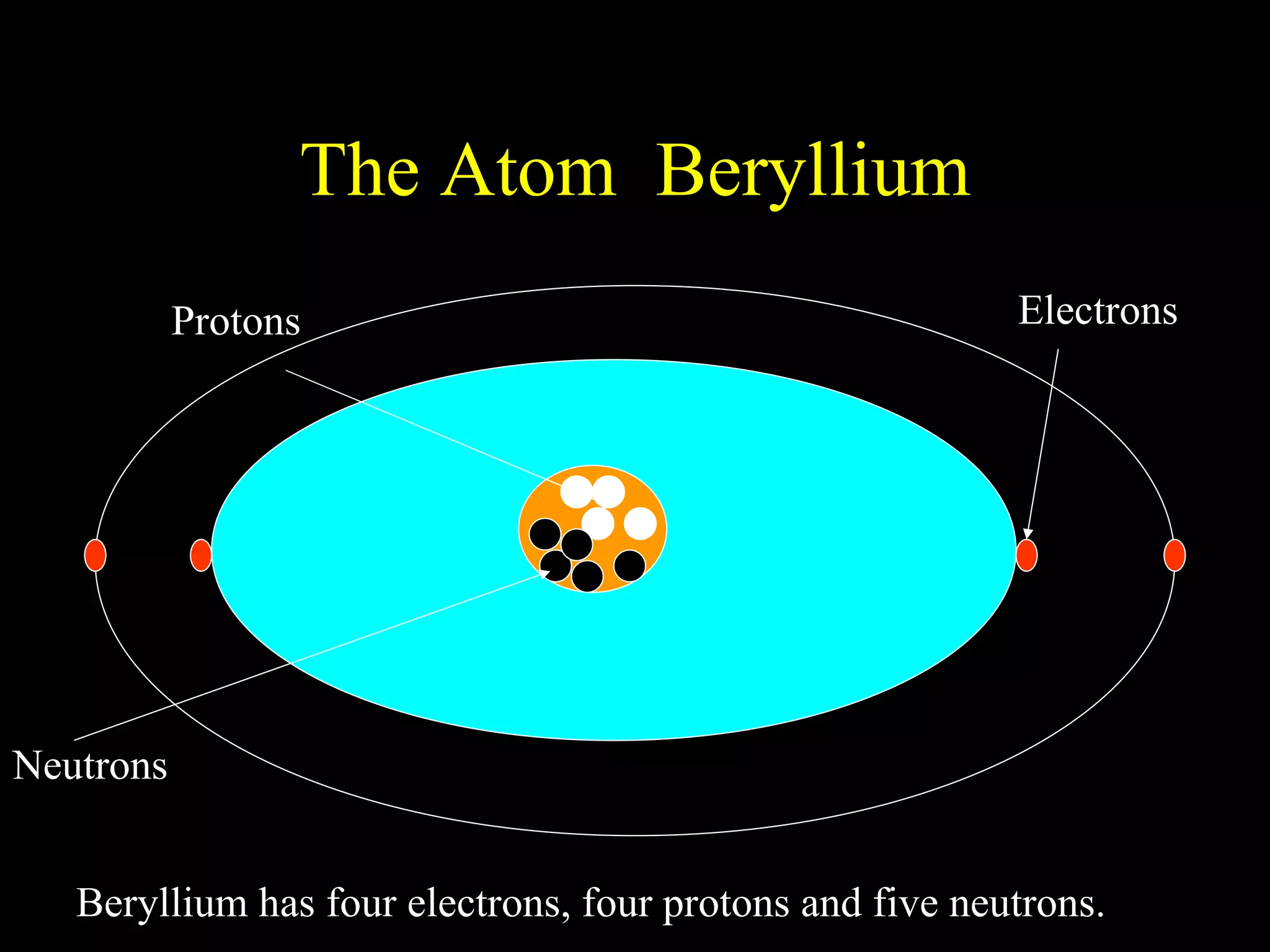
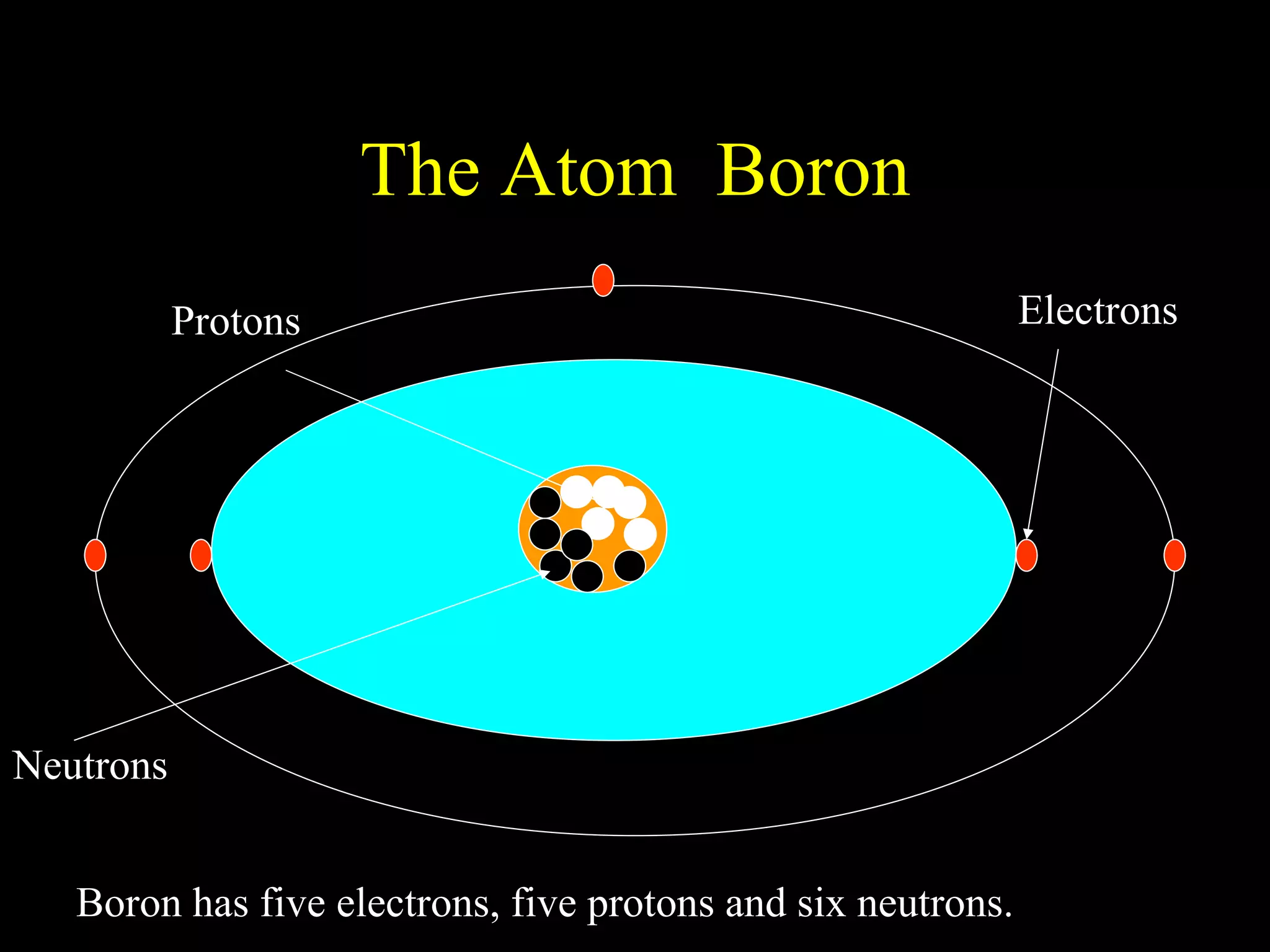
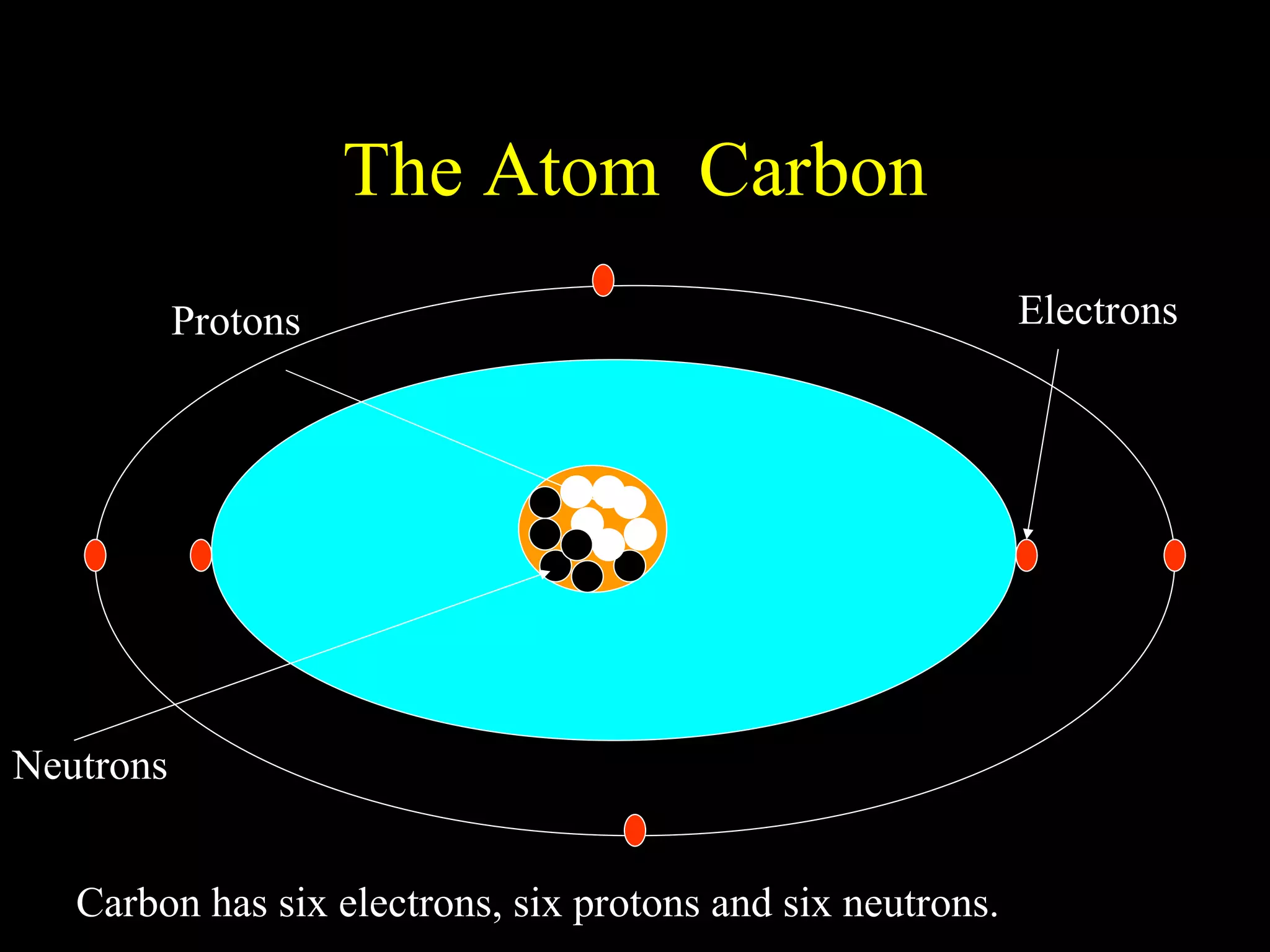
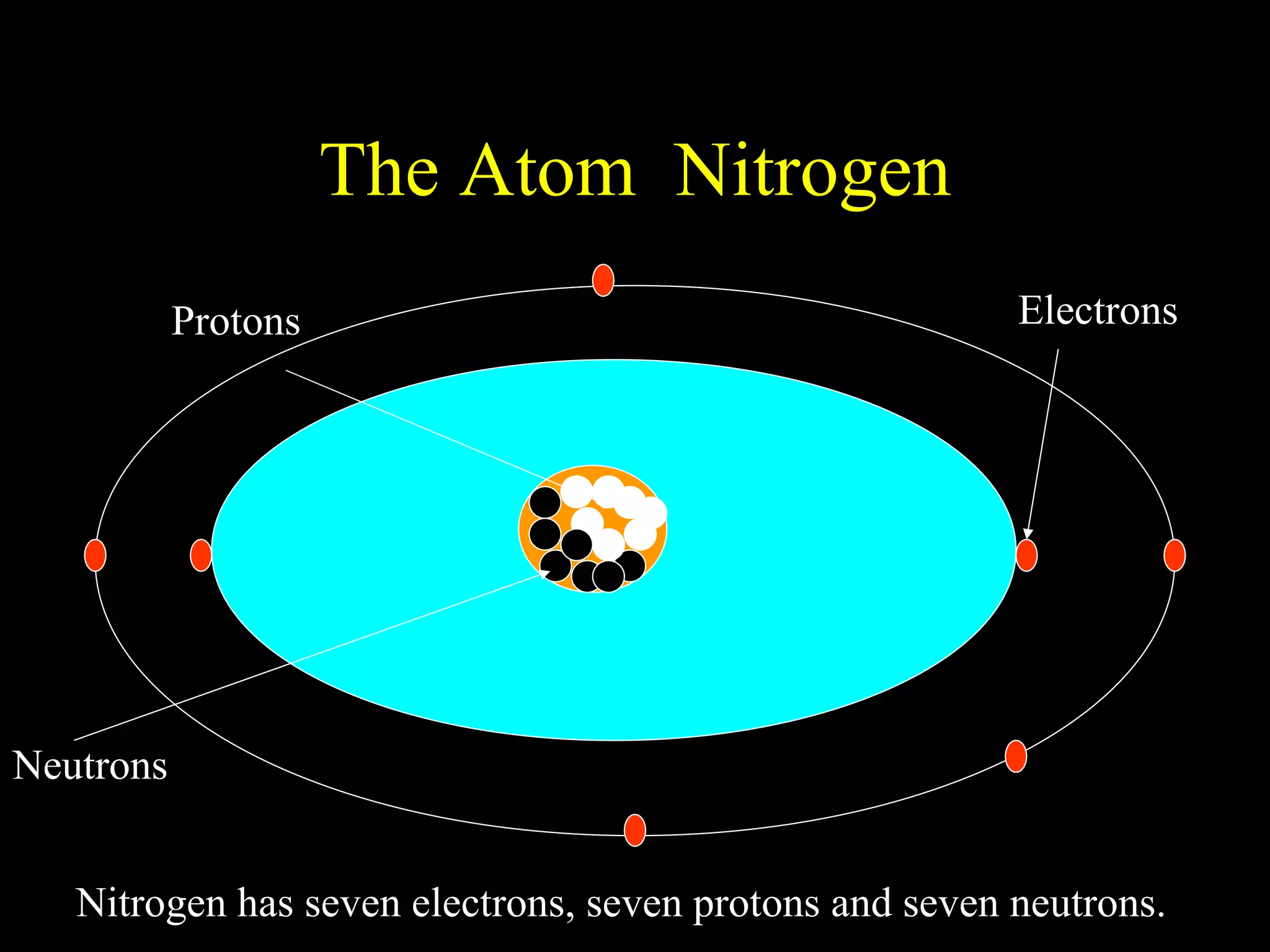
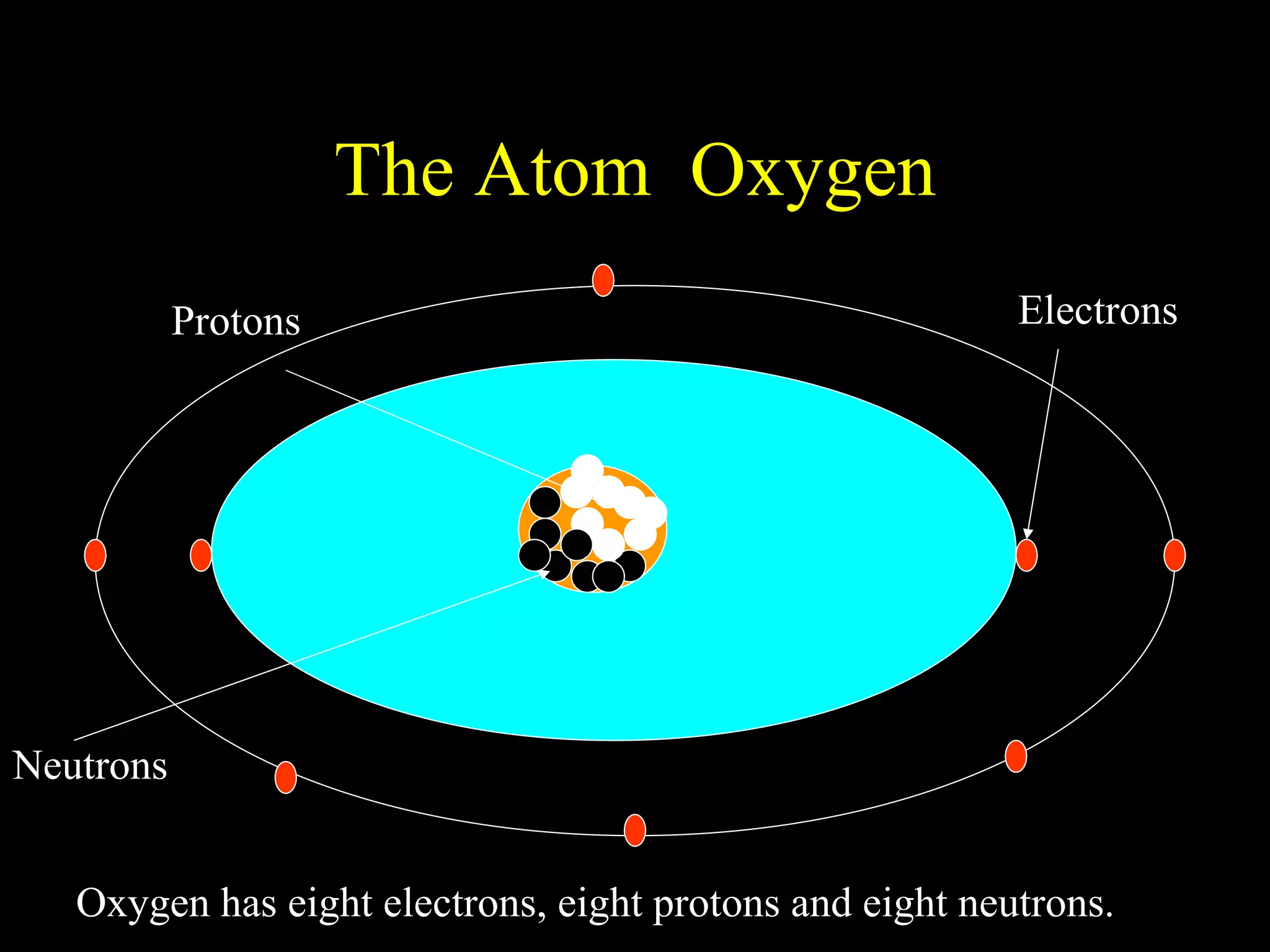
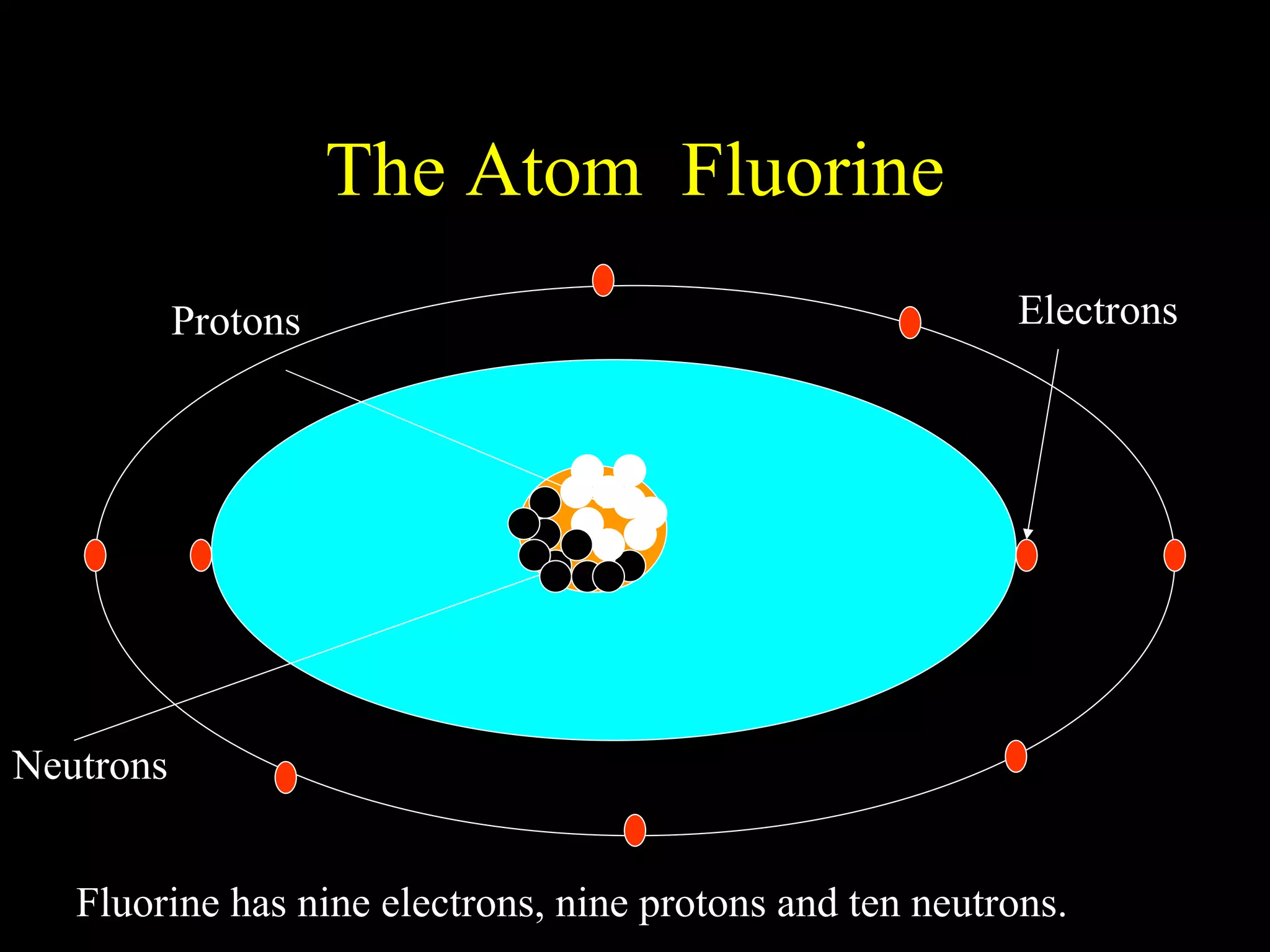
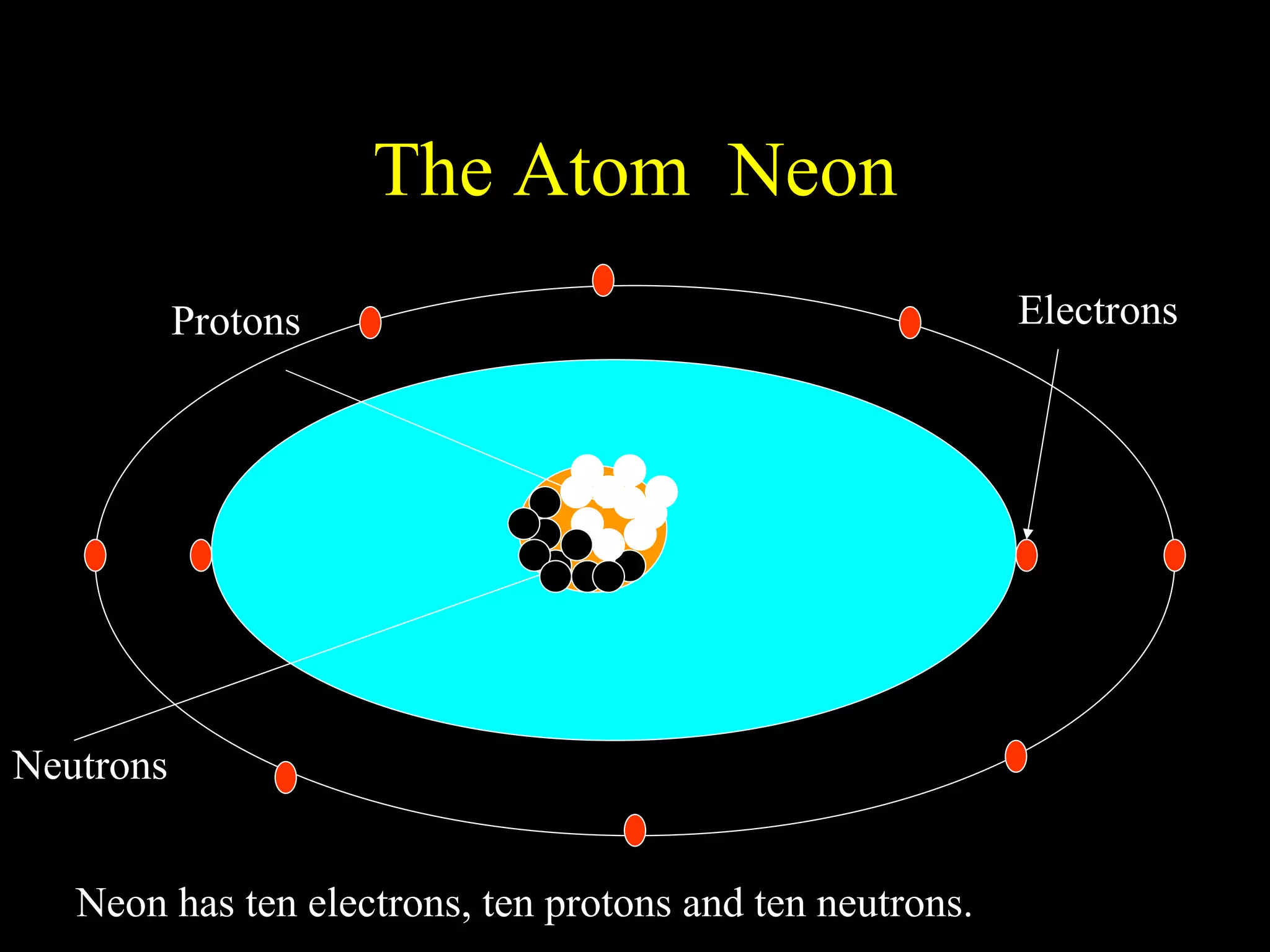
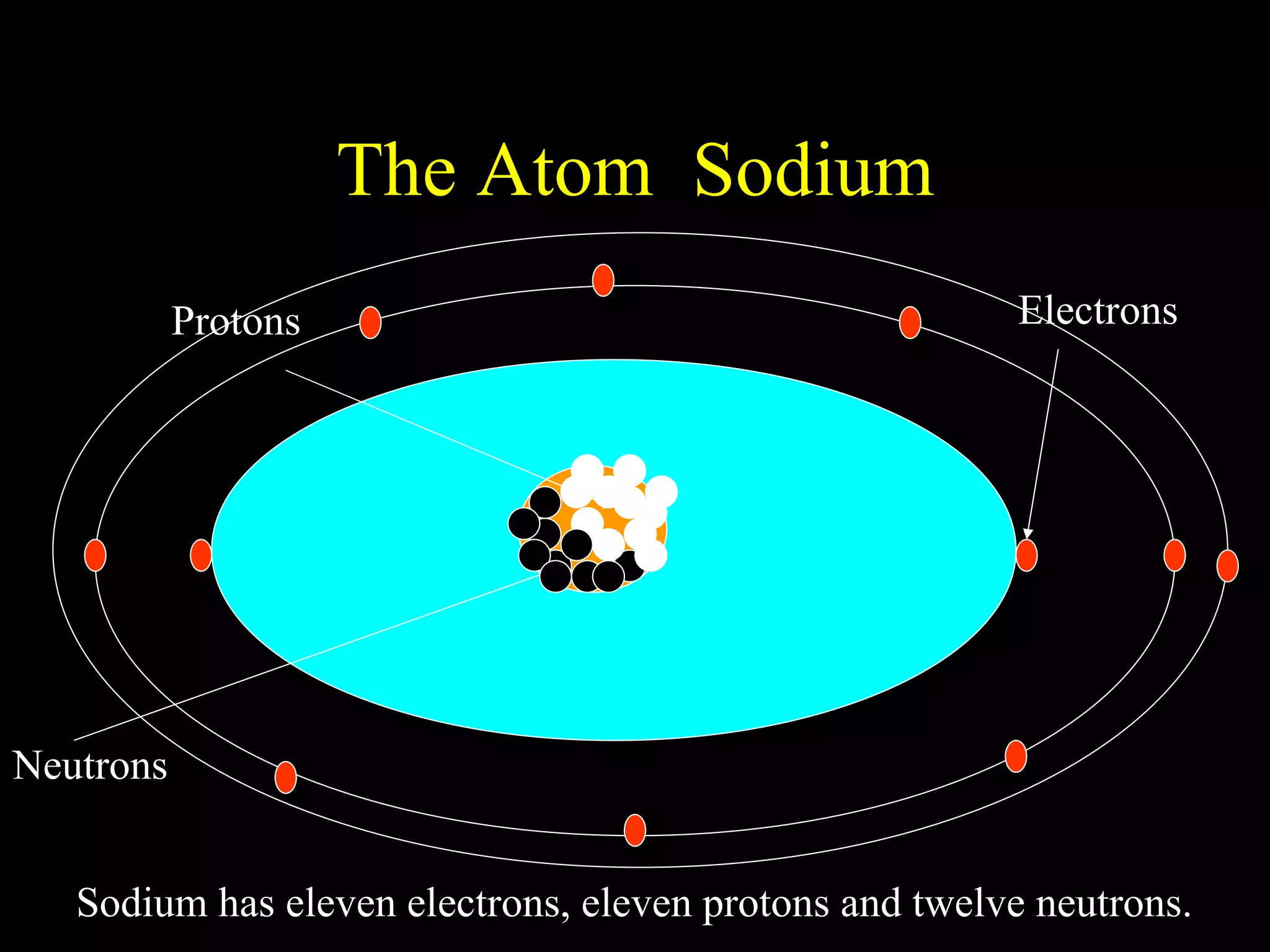
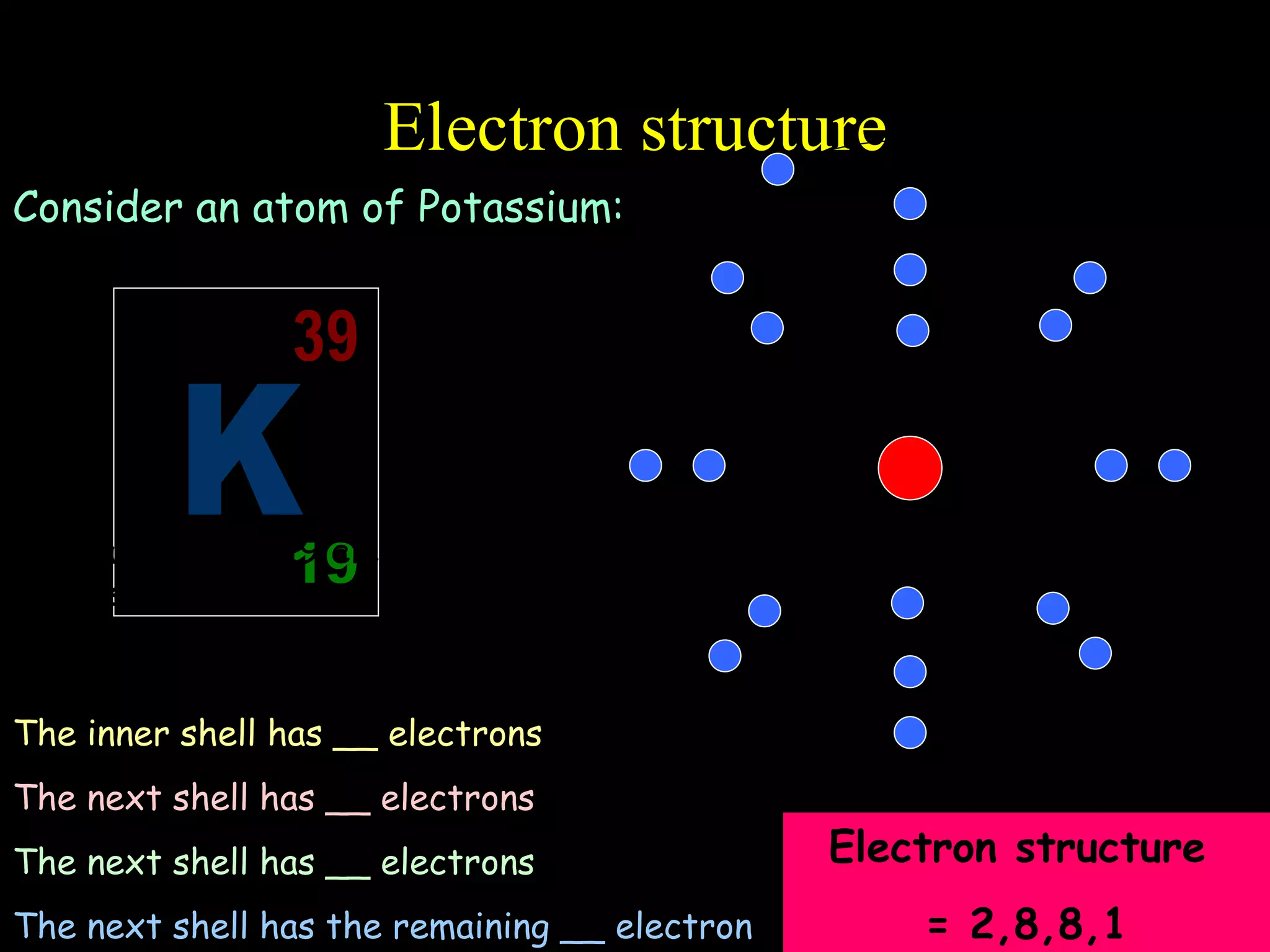
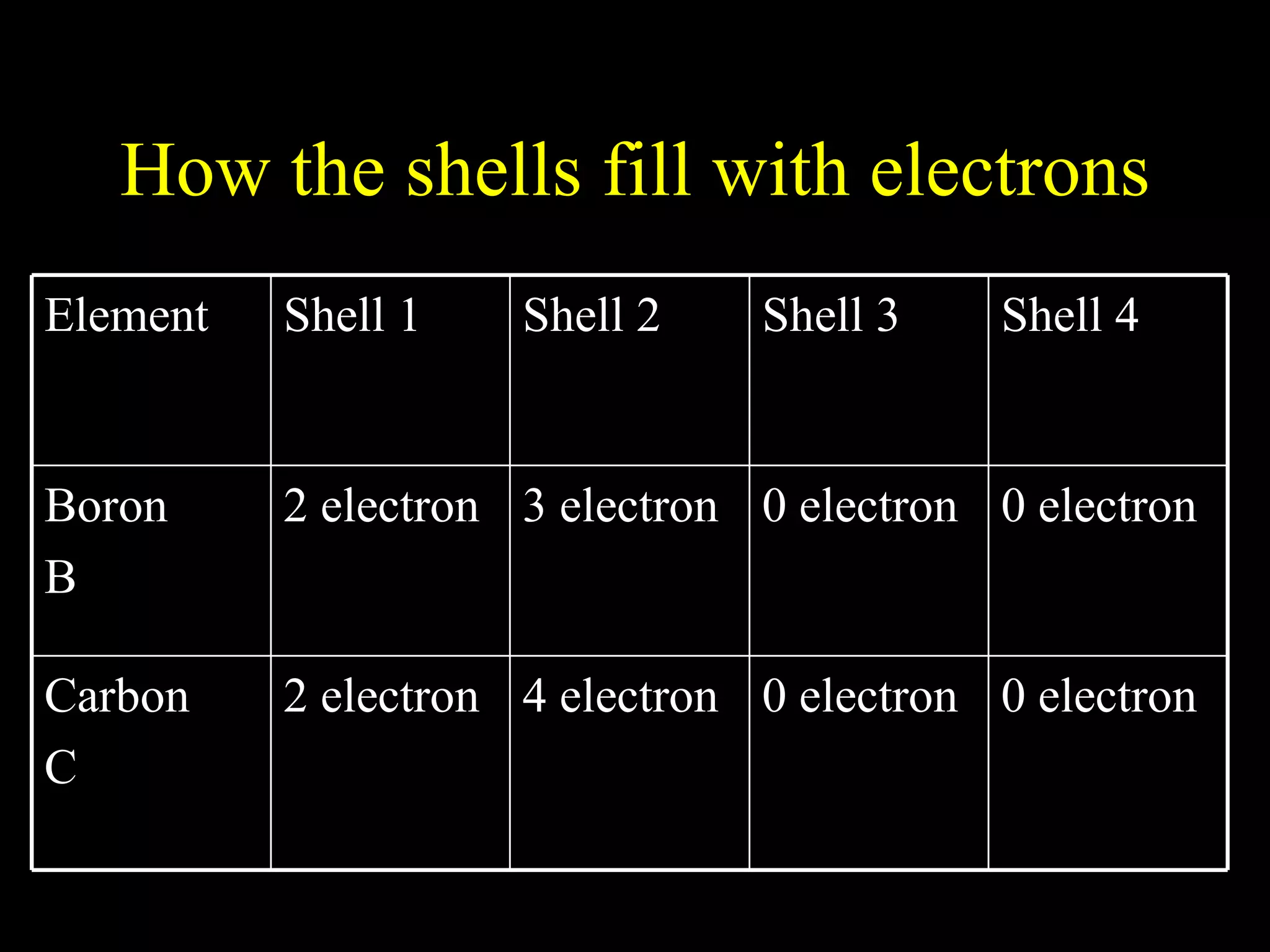
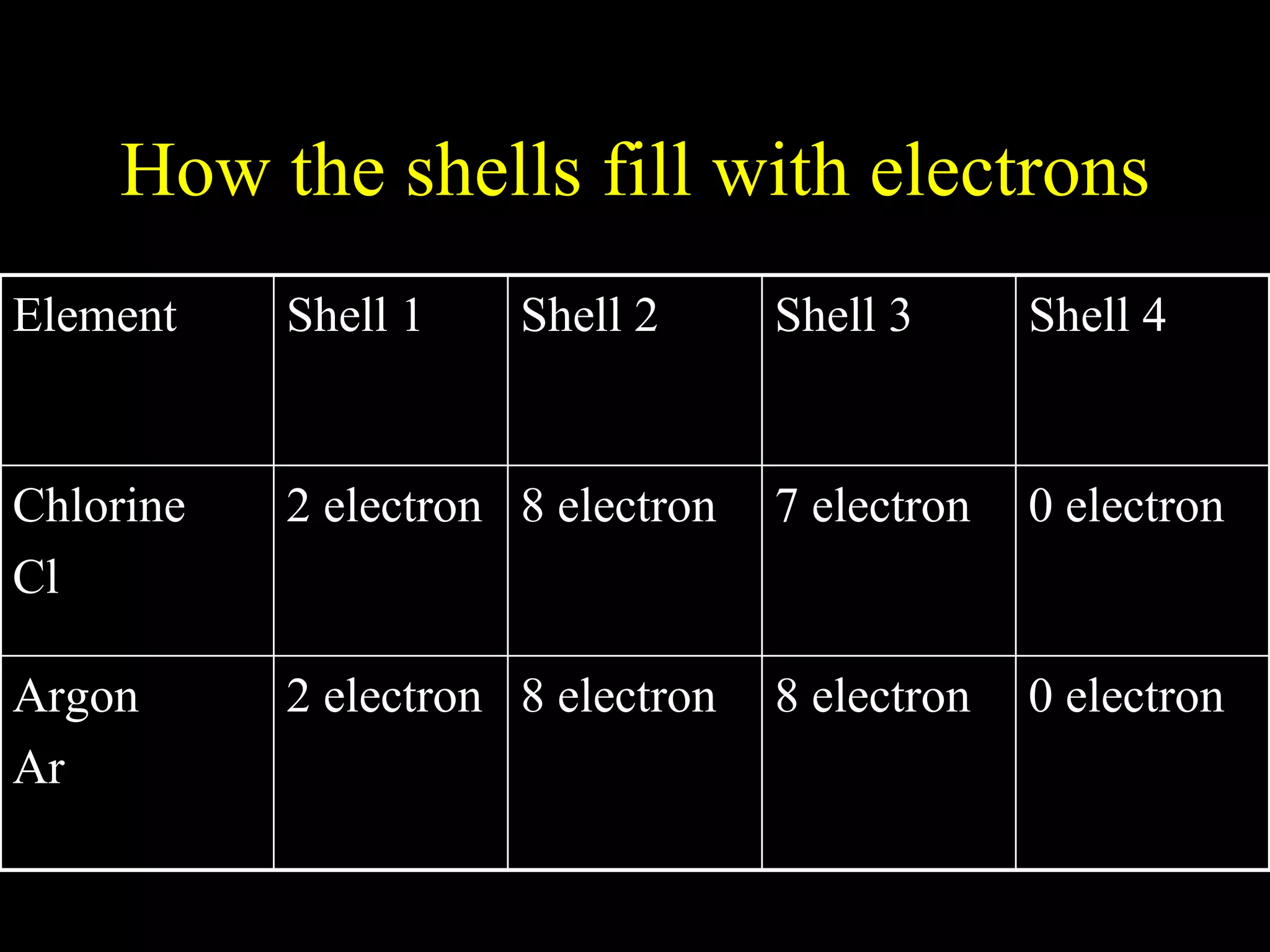
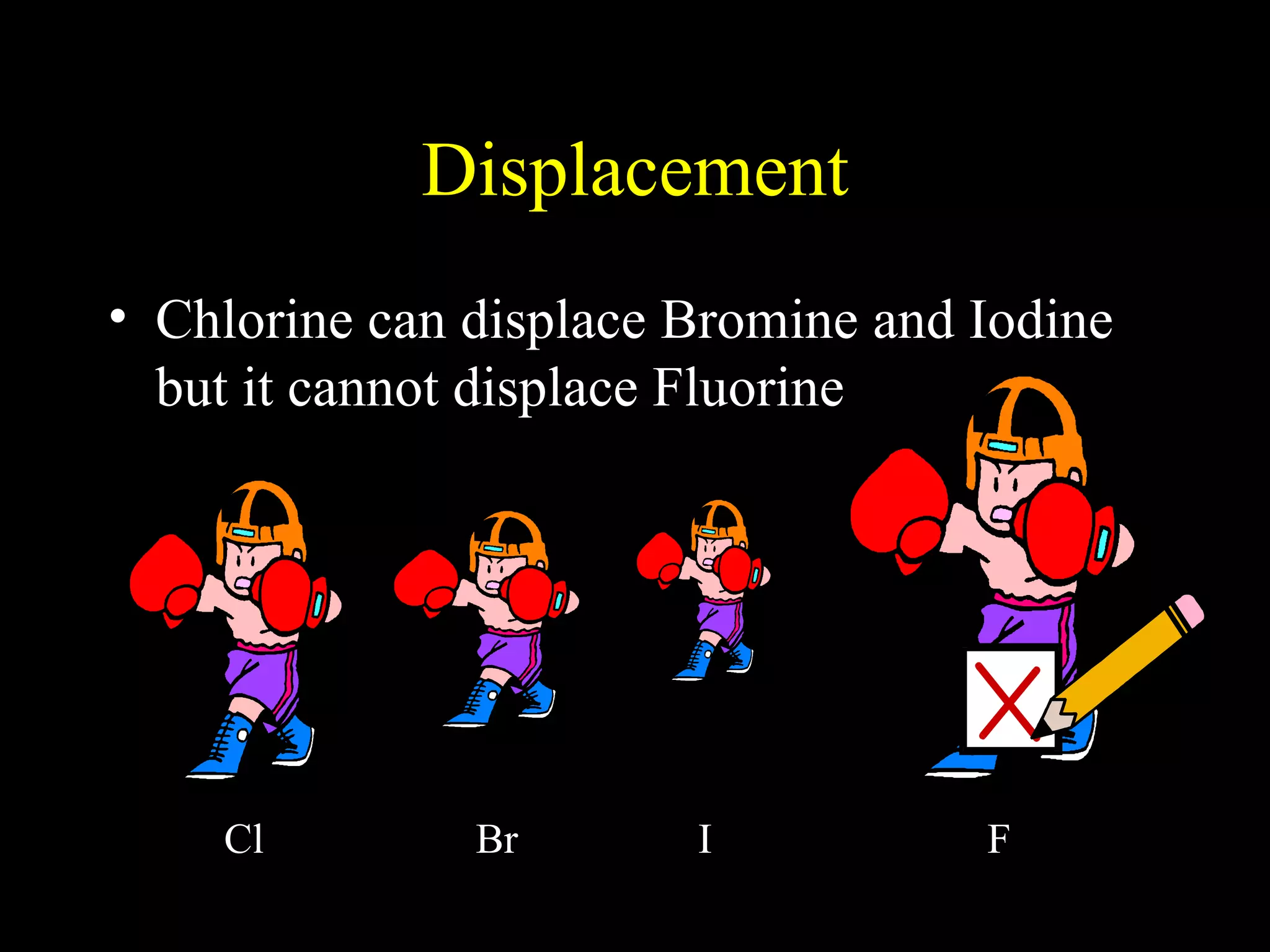
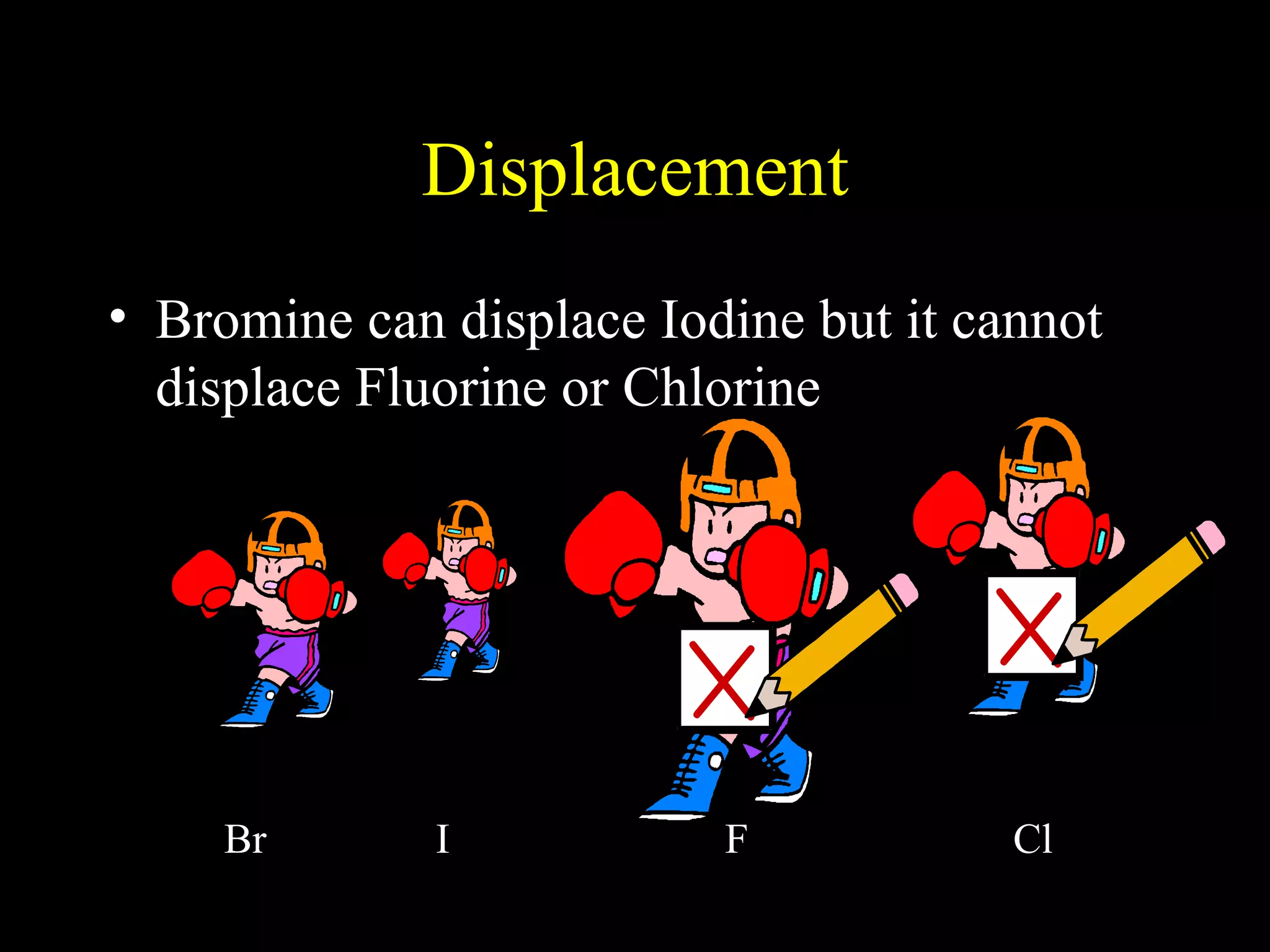
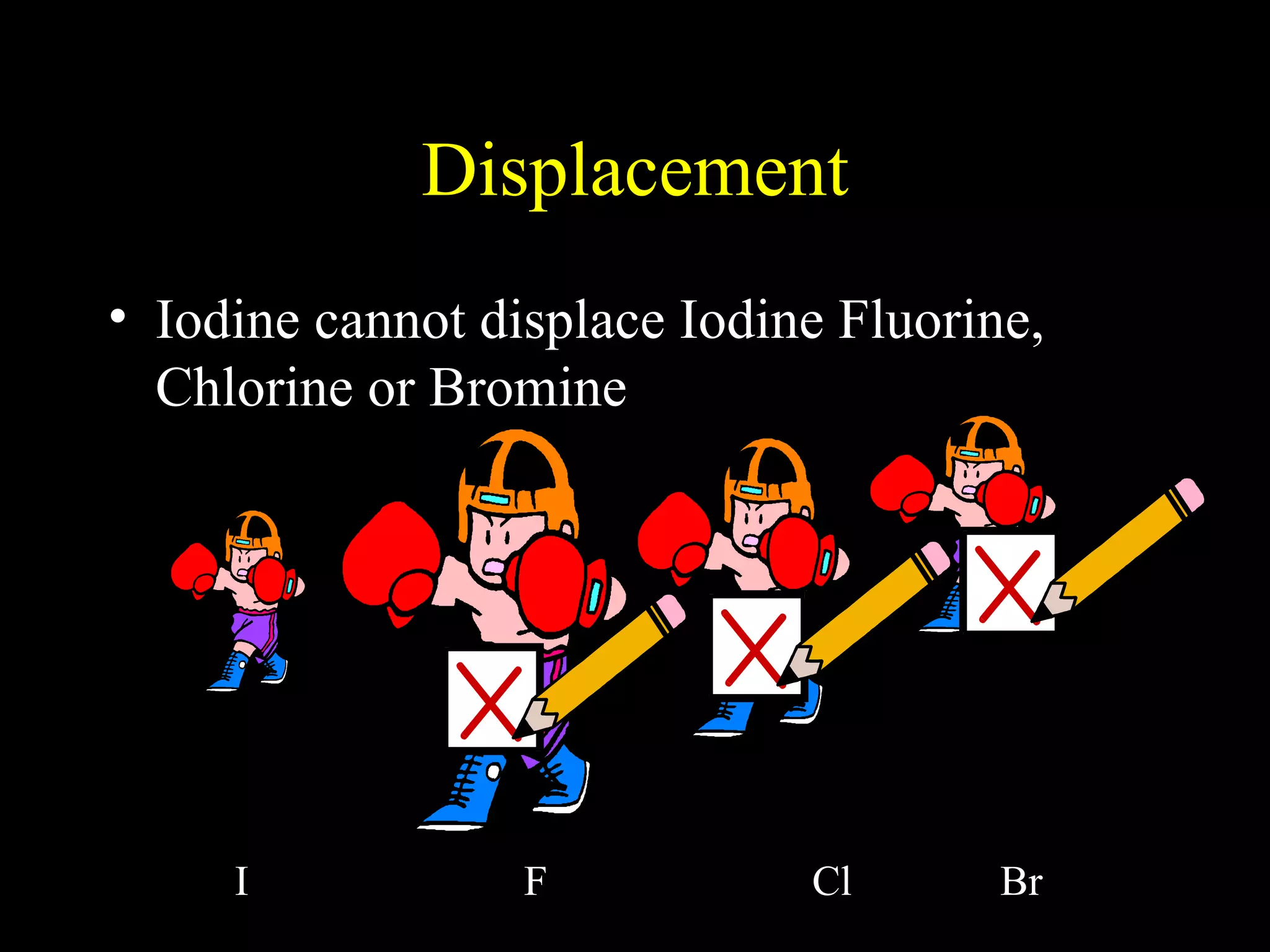
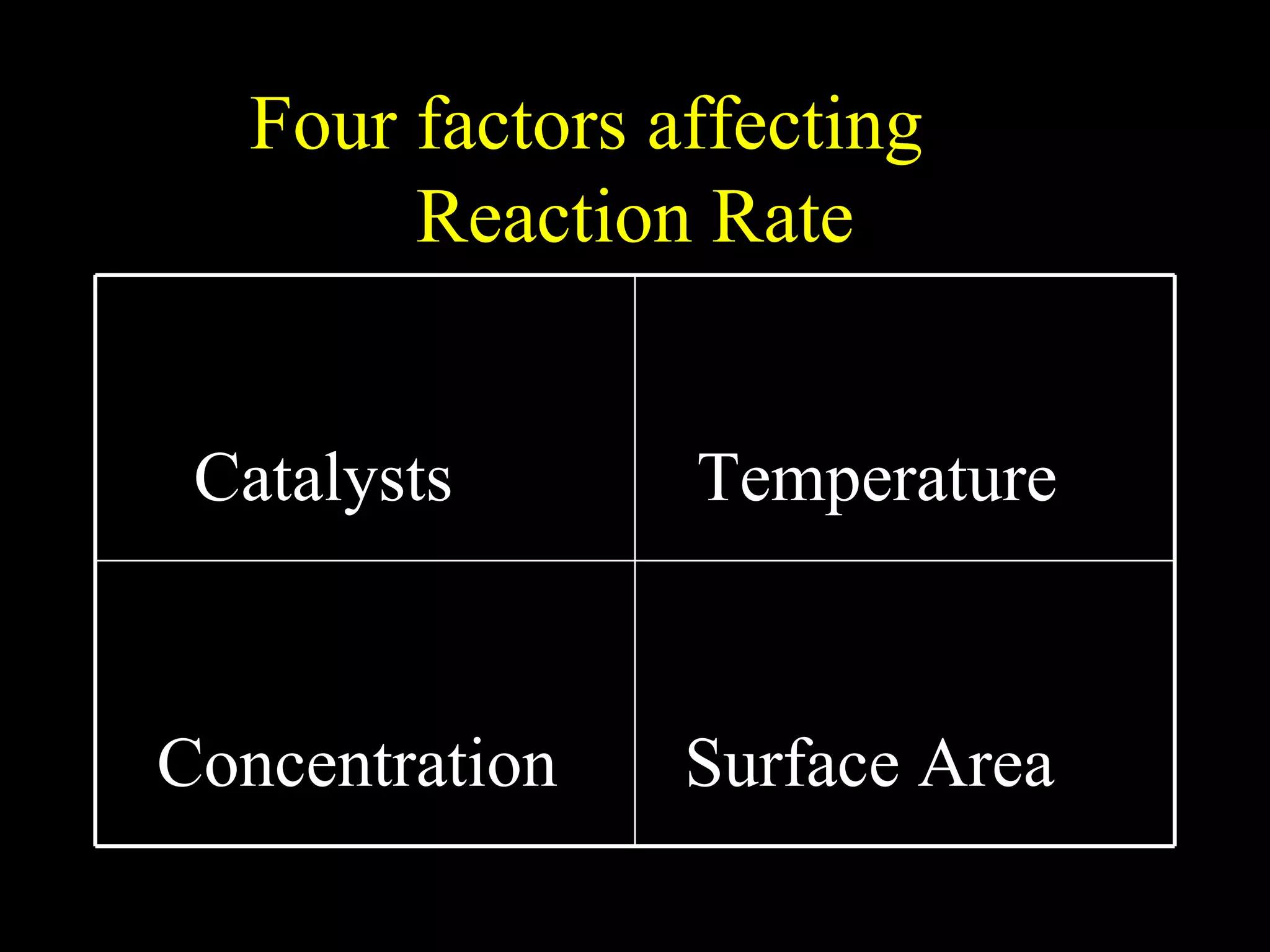
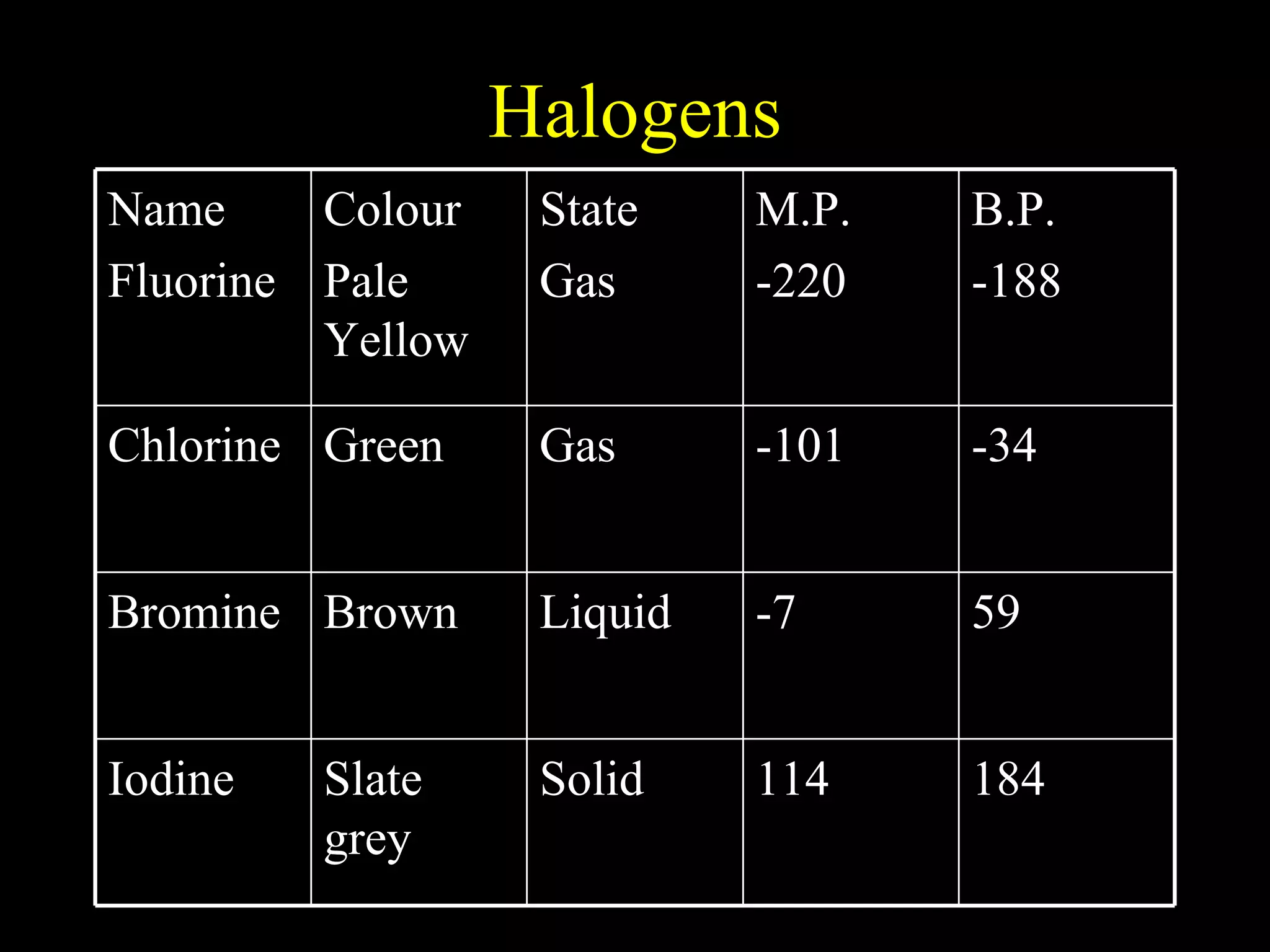
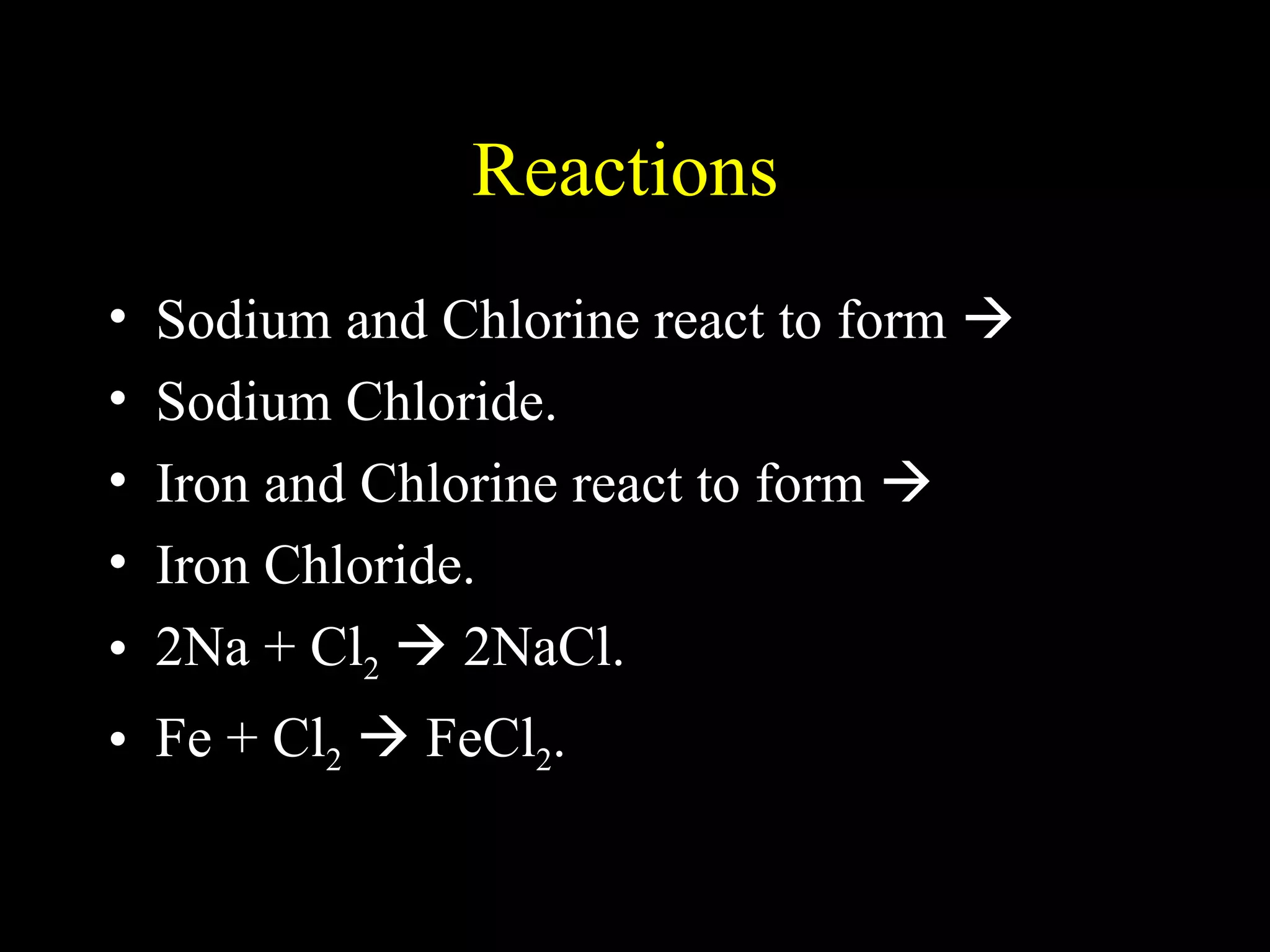
The document provides information about atomic structure including:
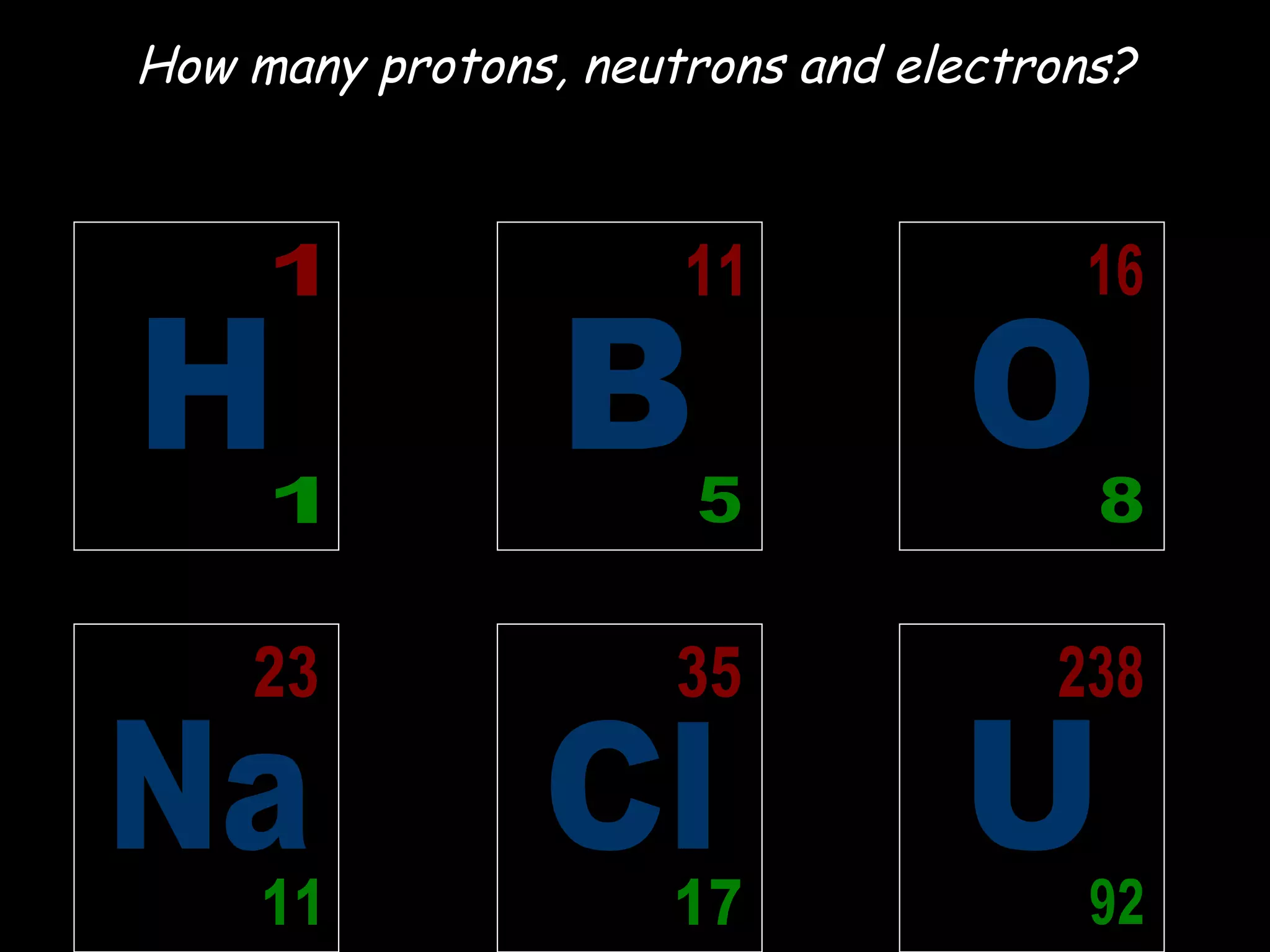
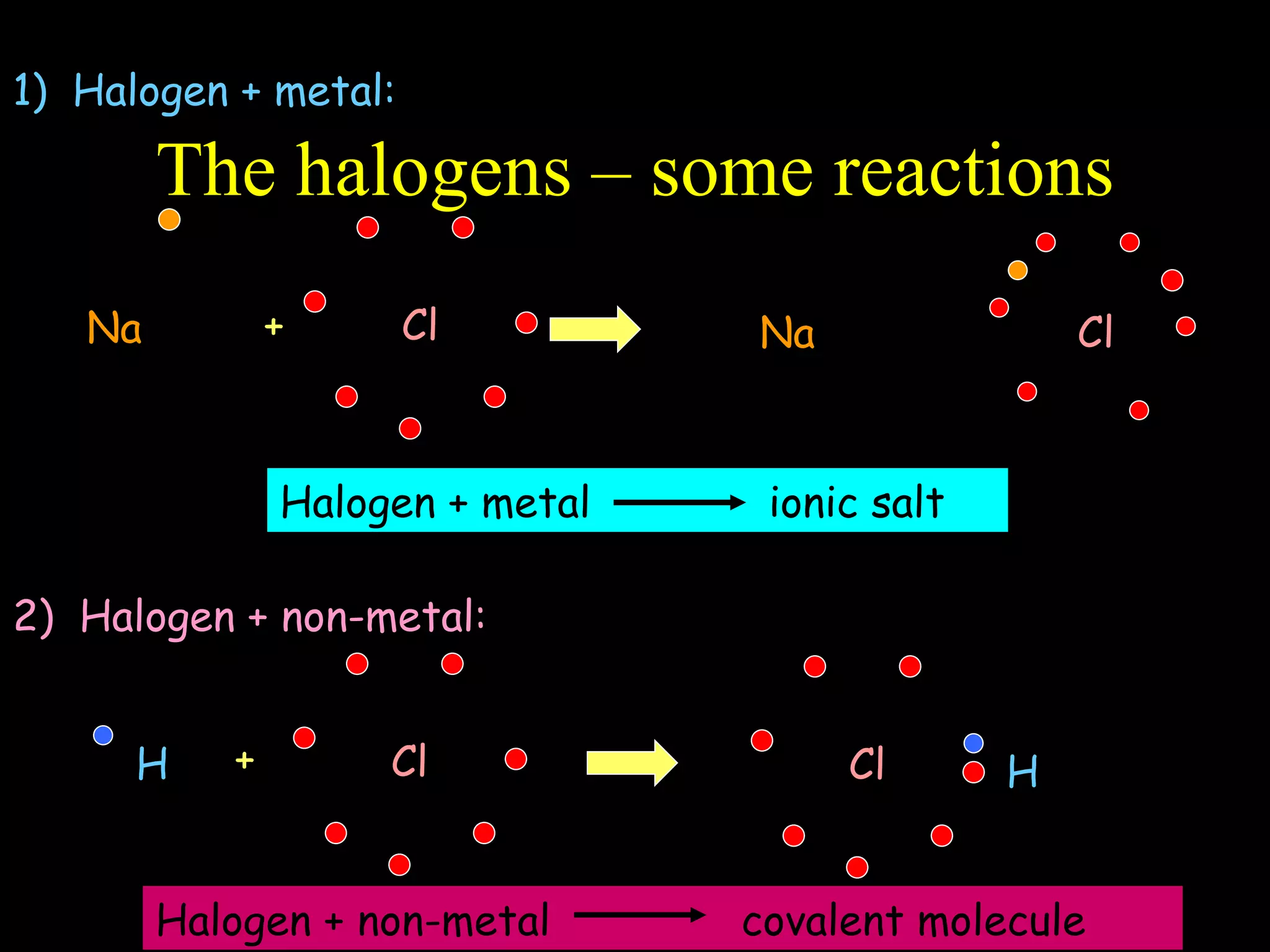
- Atoms are made up of protons, neutrons, and electrons.
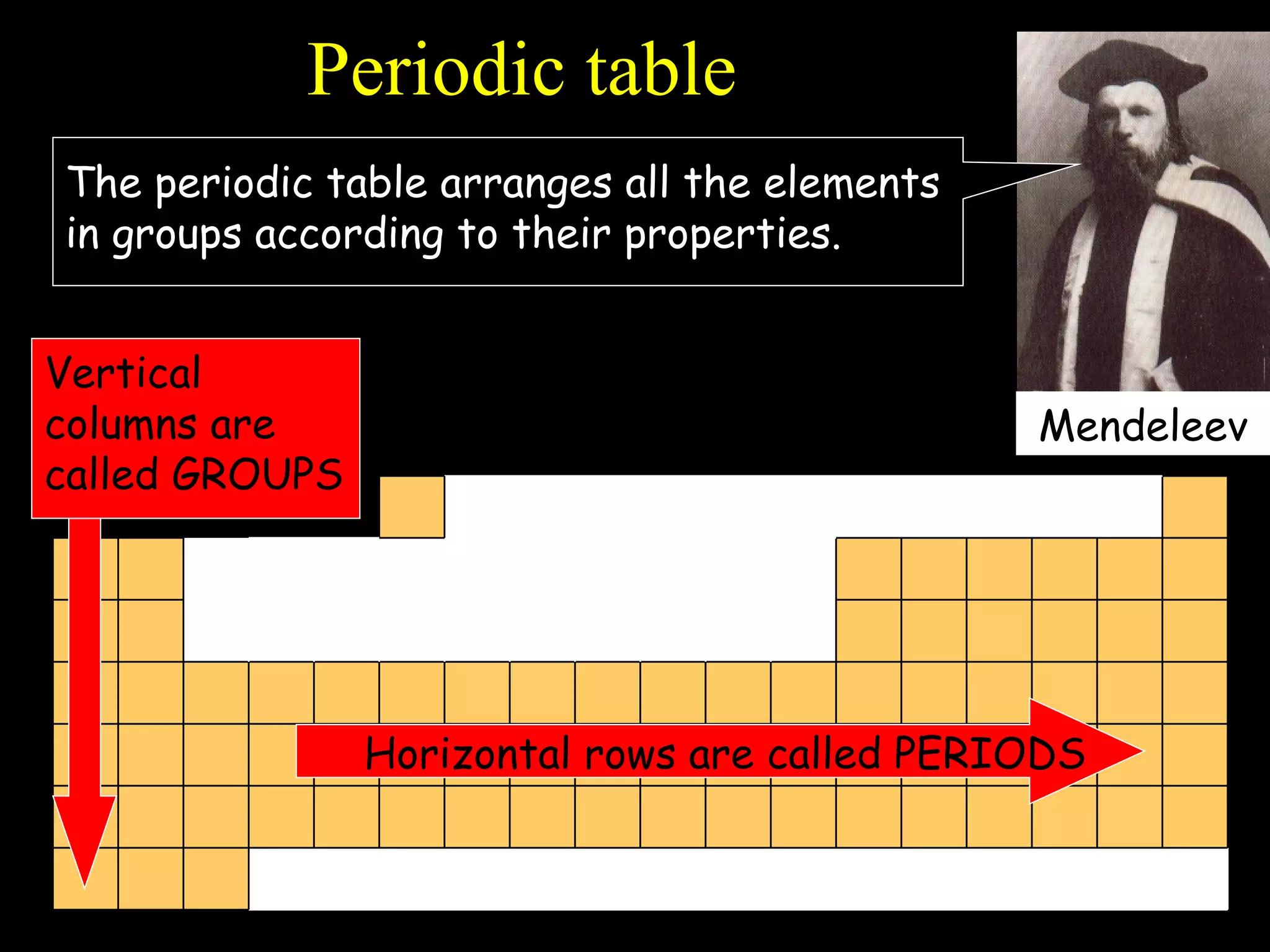
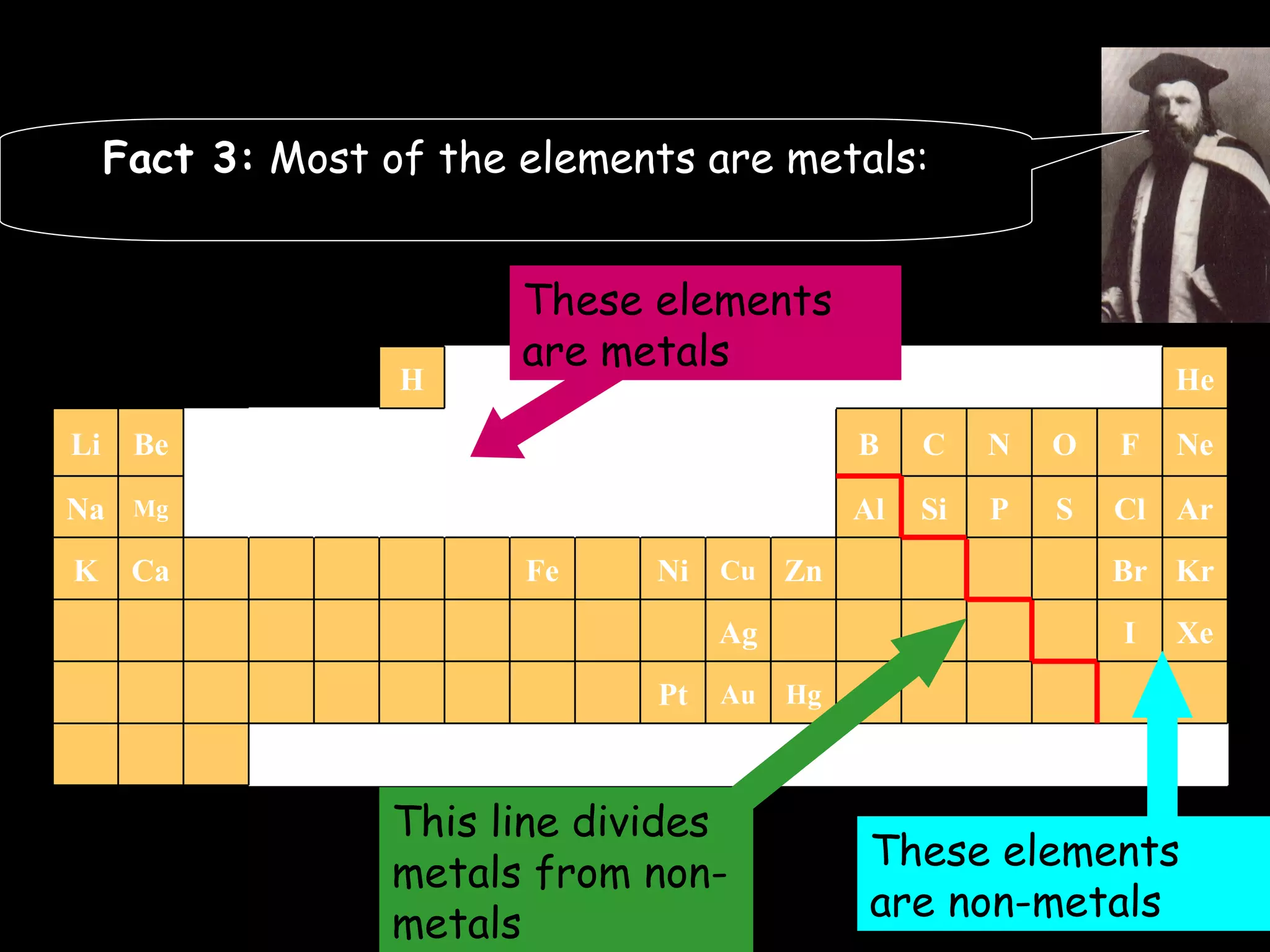
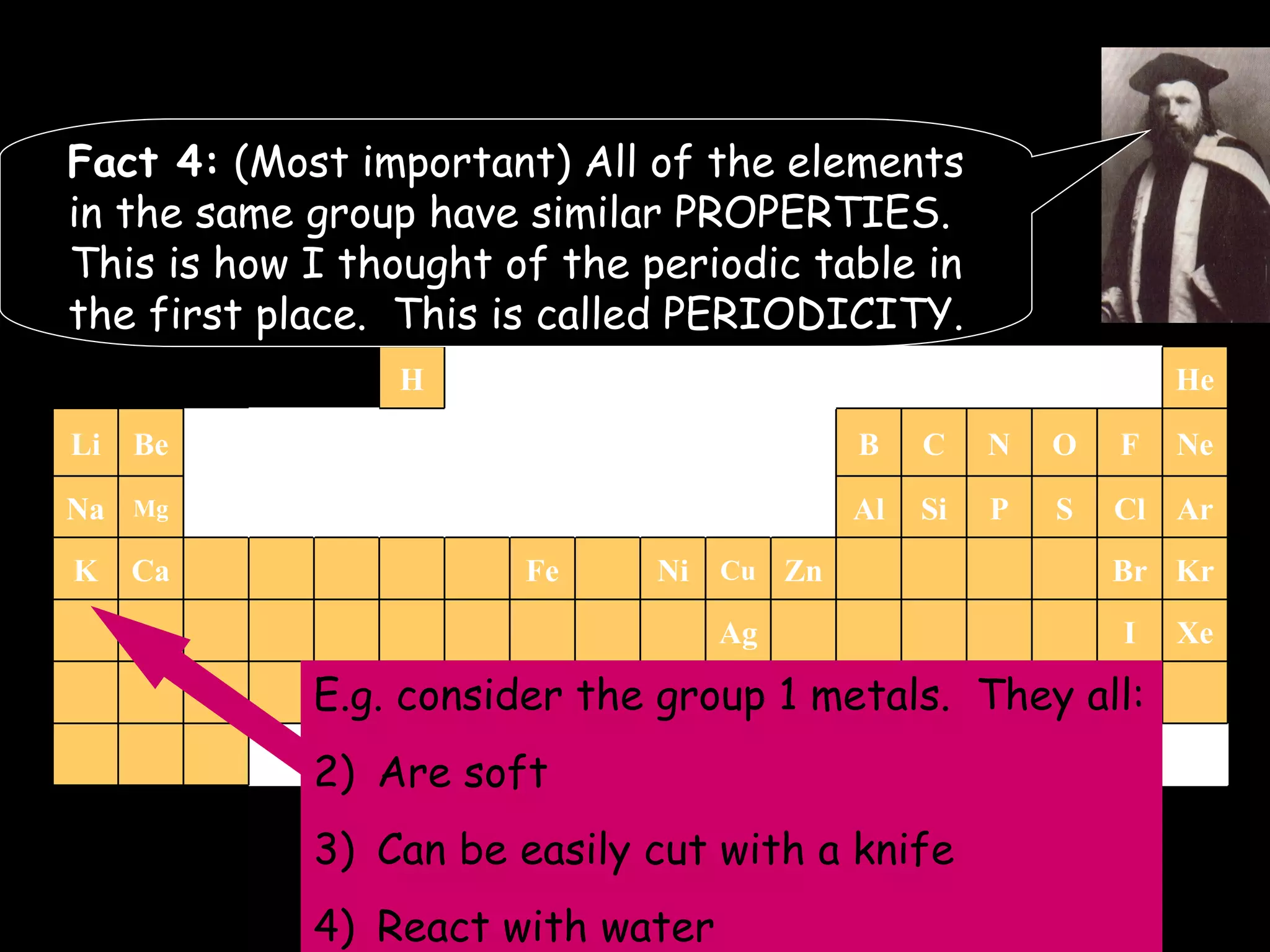
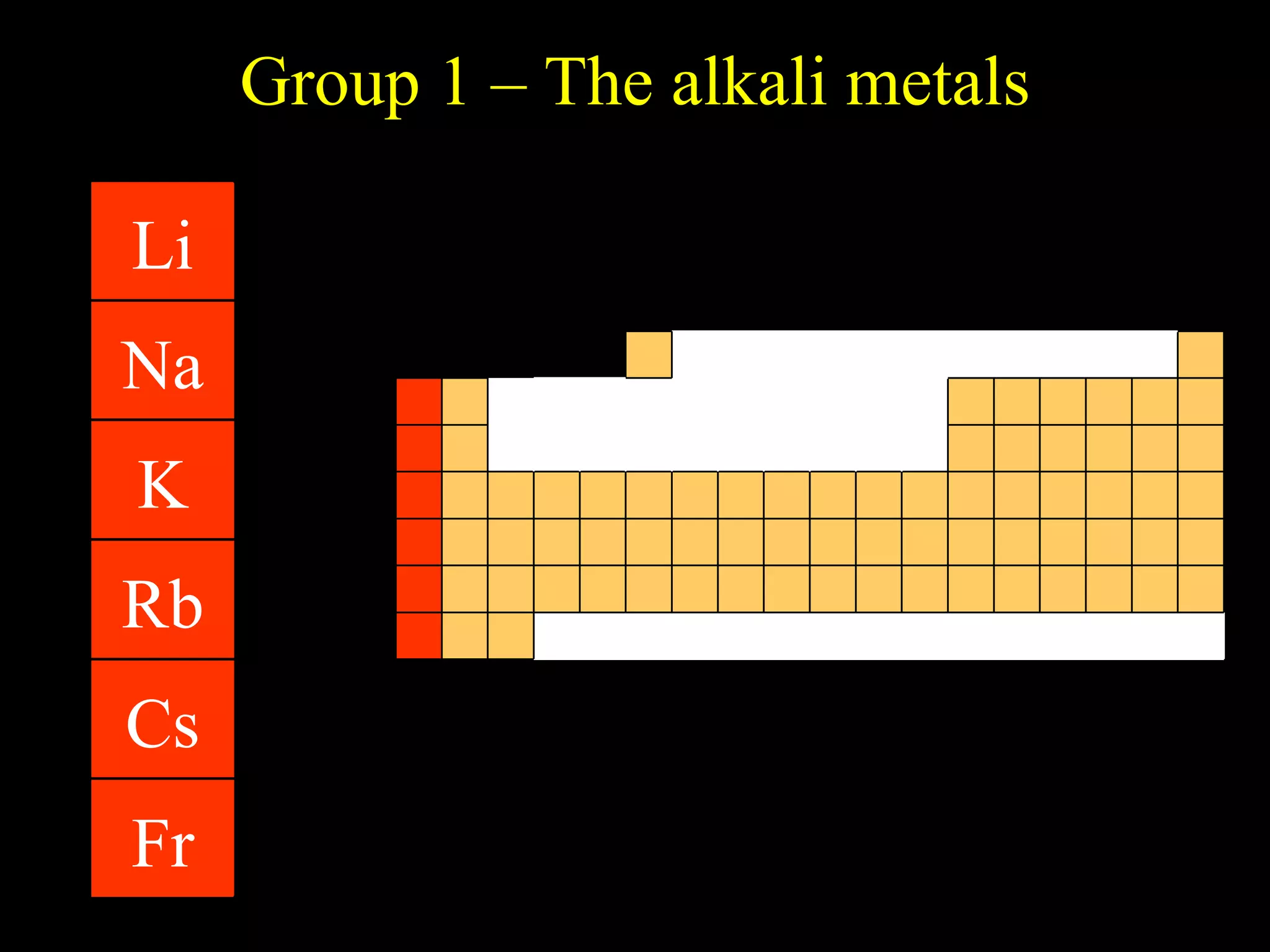
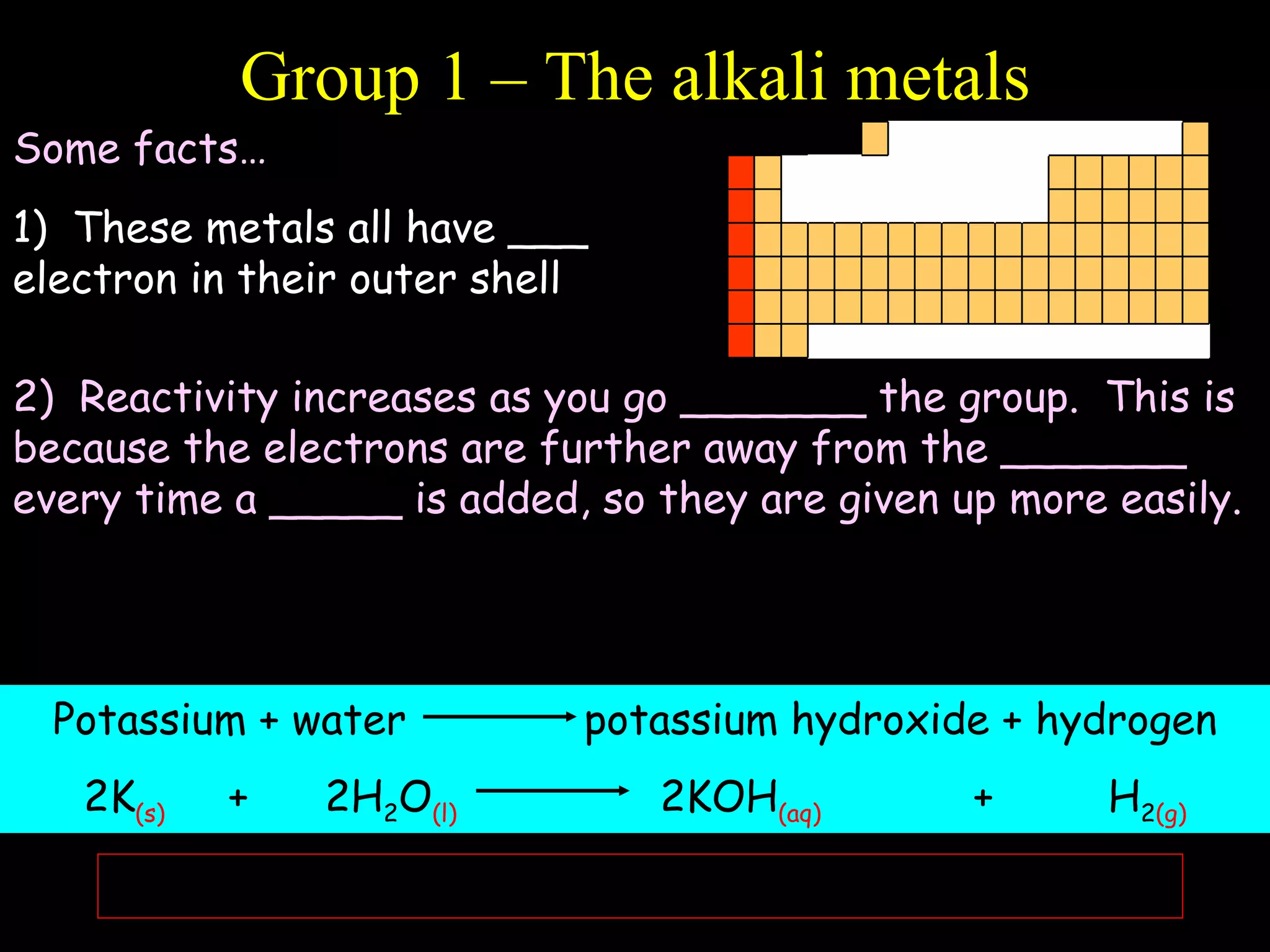
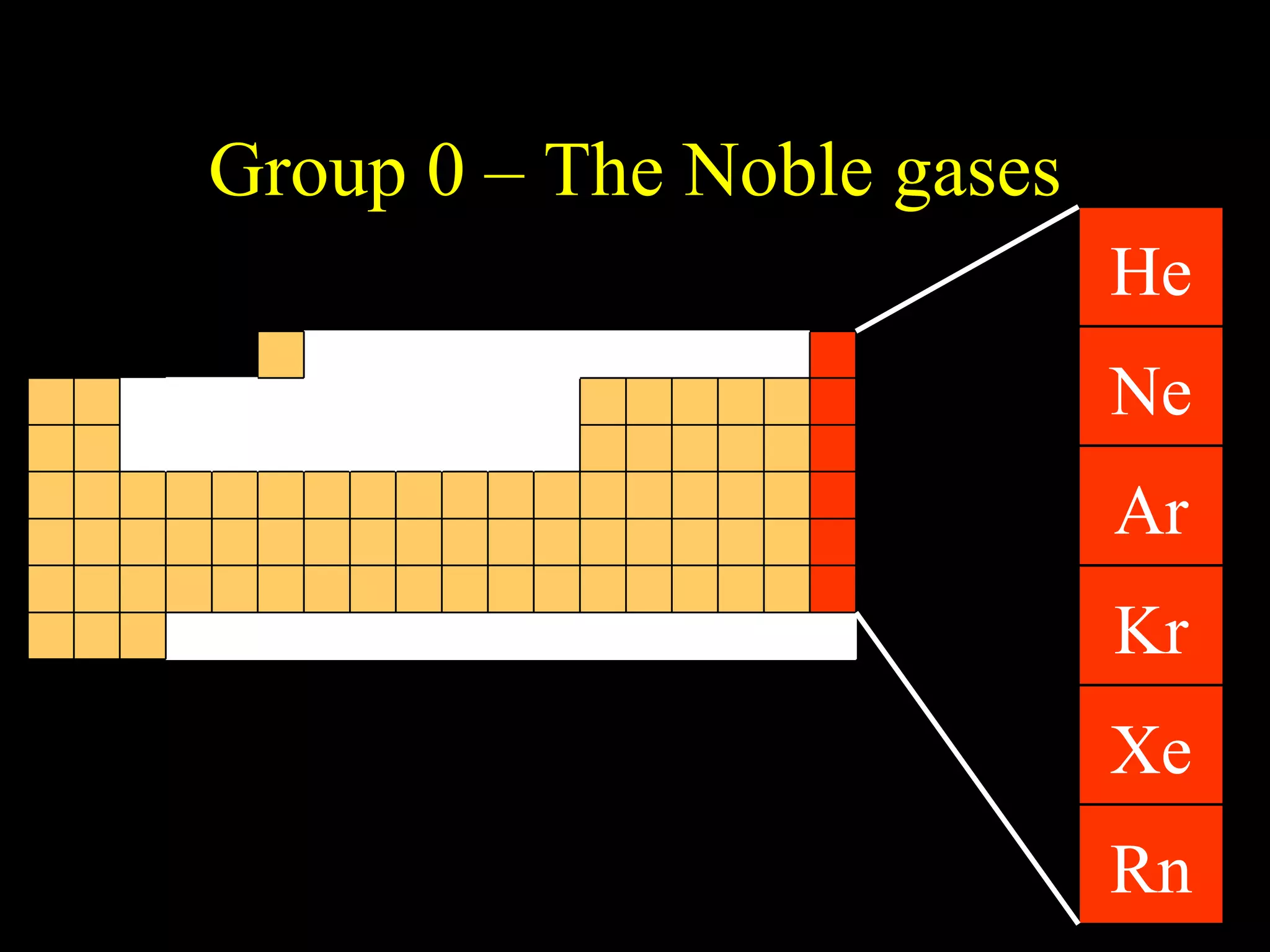
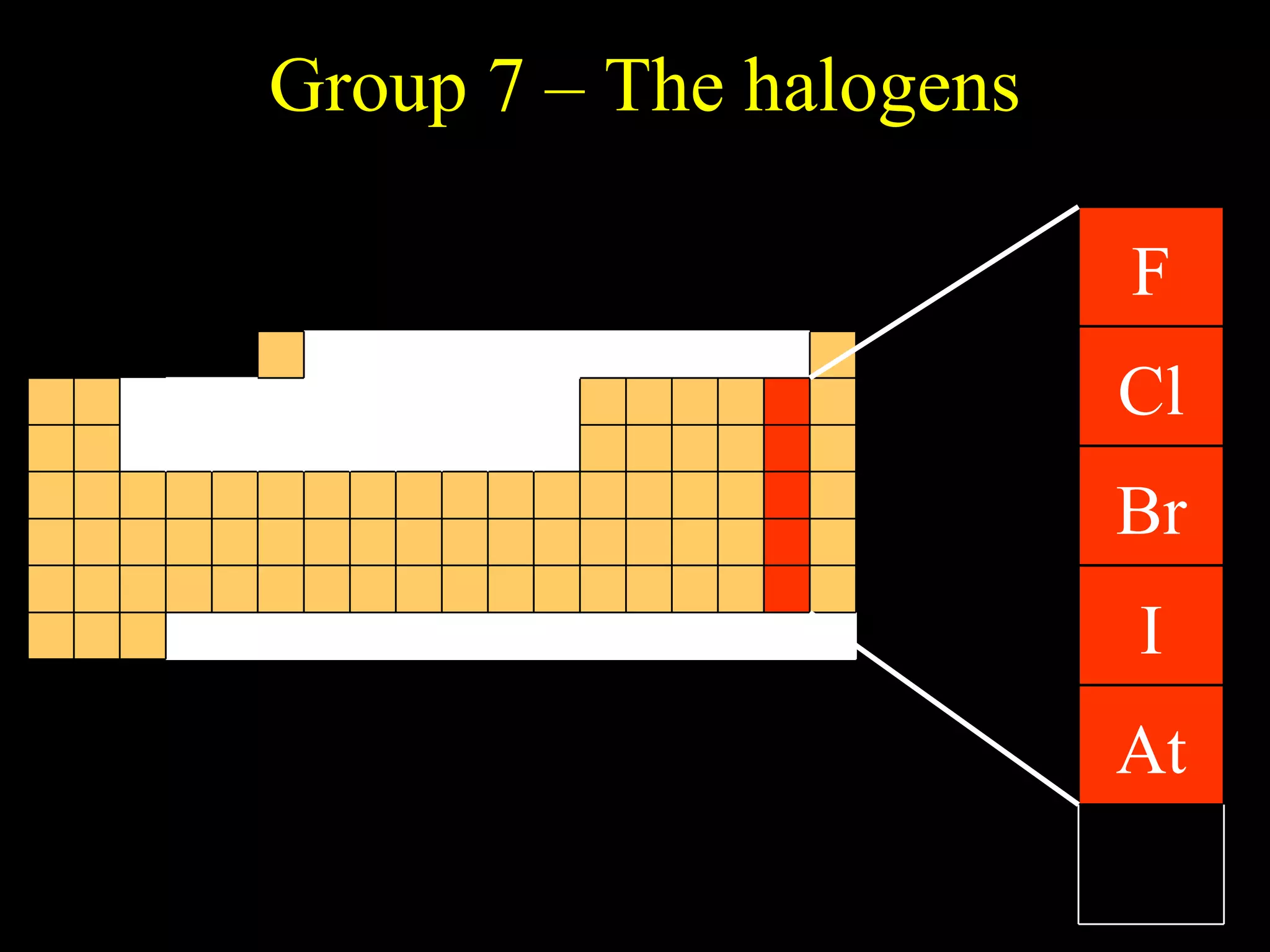
- Elements are arranged in the periodic table based on their atomic structure.
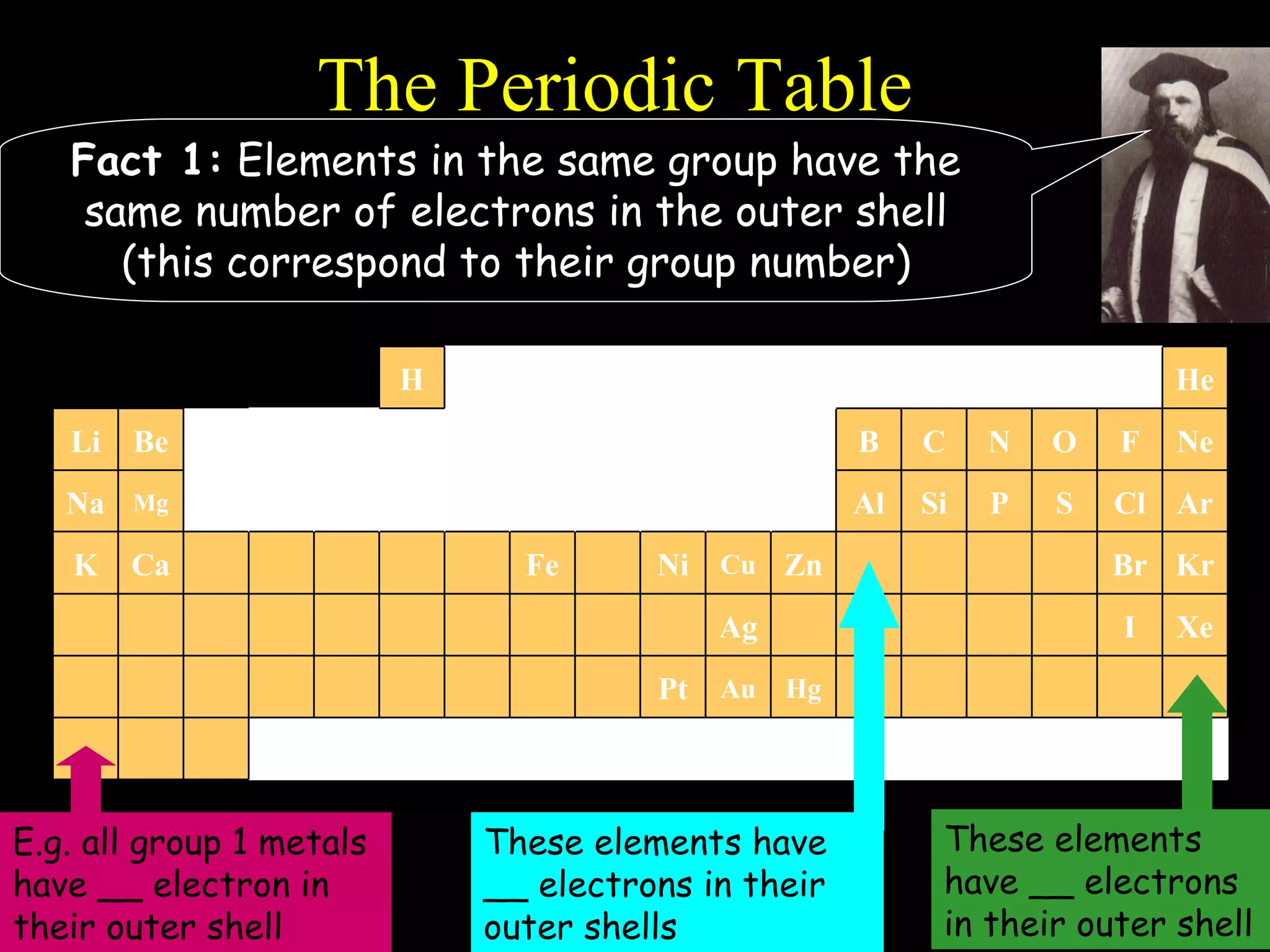
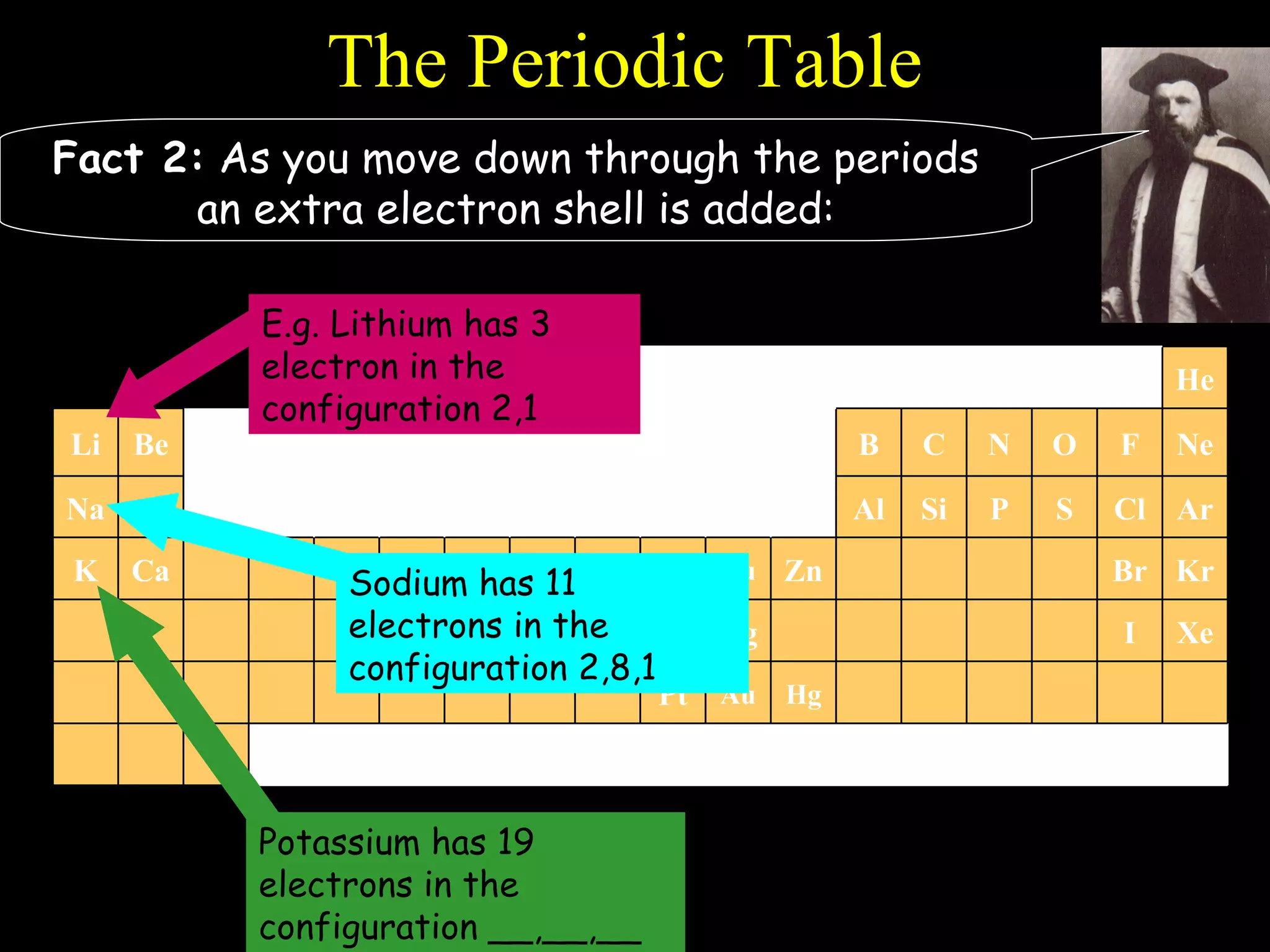
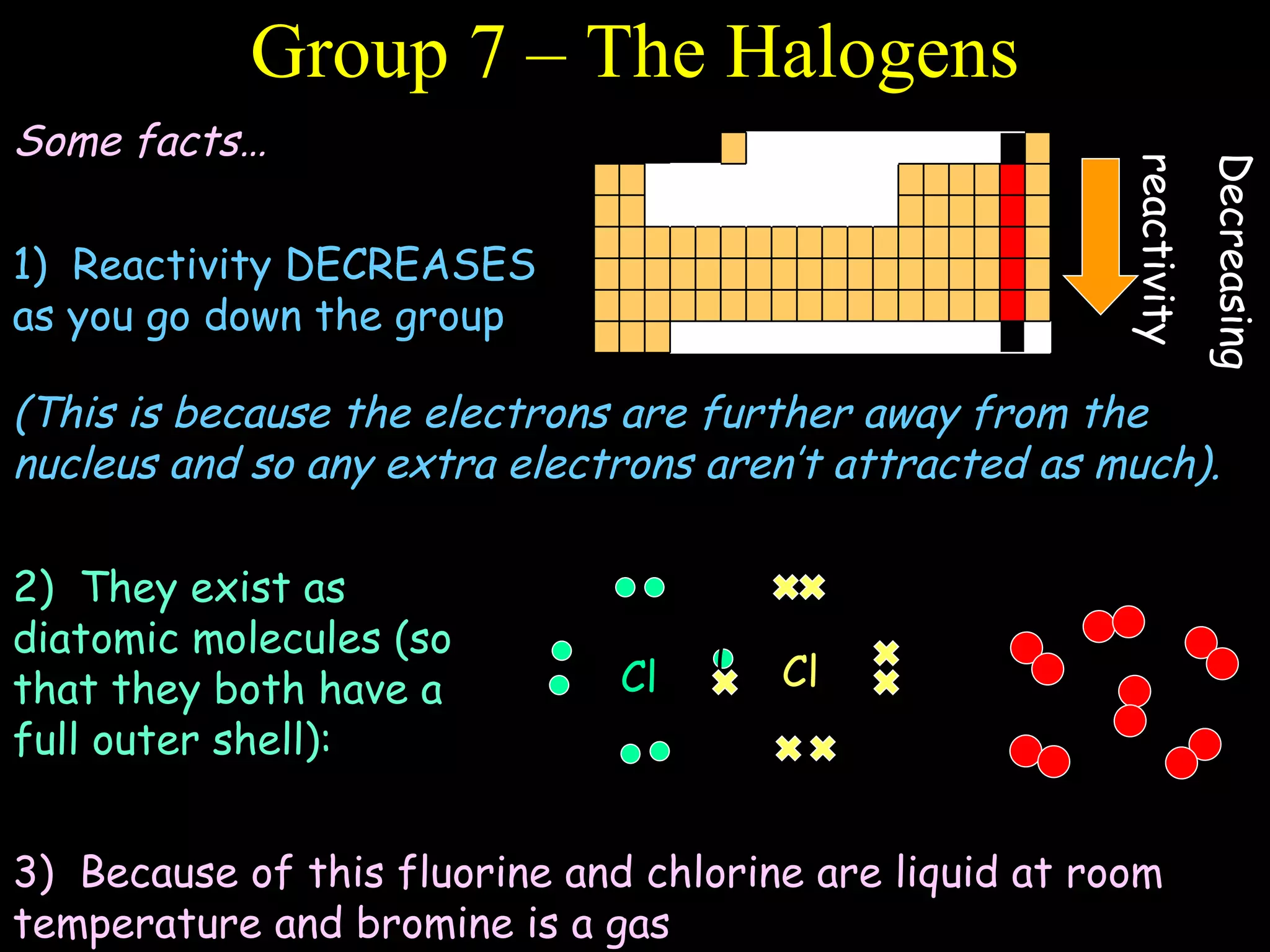
- The periodic table is organized into periods and groups based on similarities in electrons and properties.
- Electrons fill atomic orbitals according to specific rules, with the first shell holding up to 2 electrons and subsequent shells holding up to 8 electrons each.