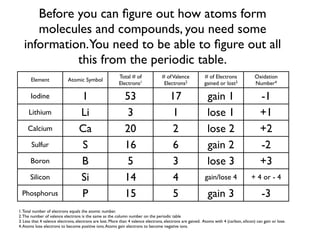
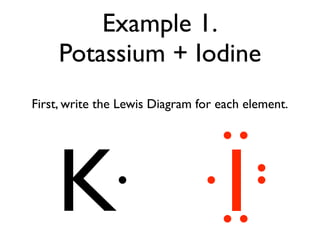
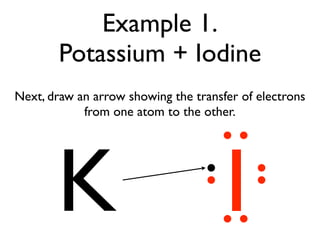
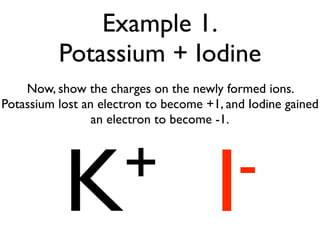
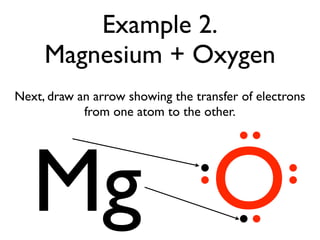
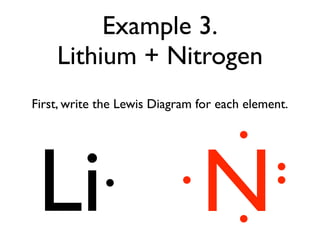
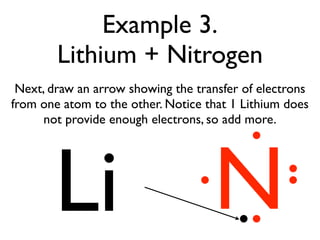
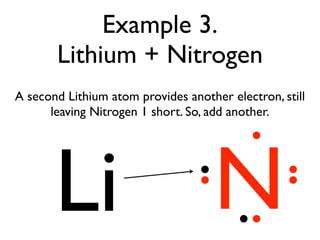
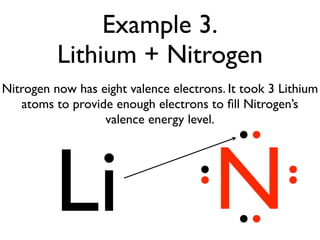
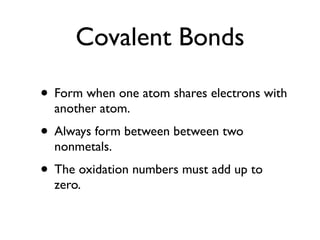
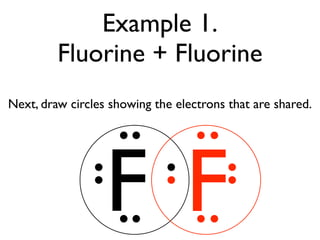
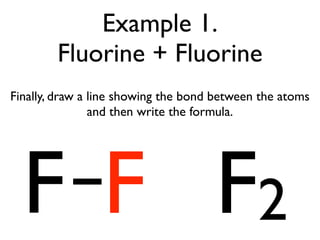
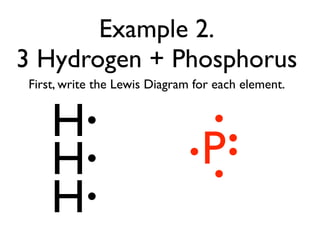
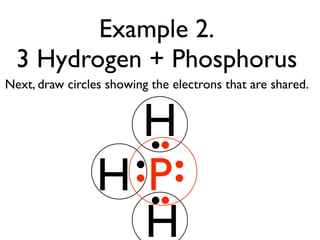
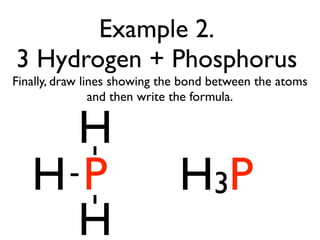
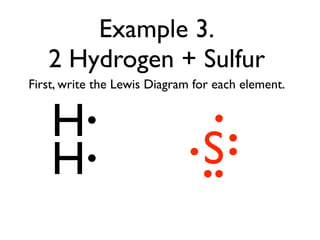
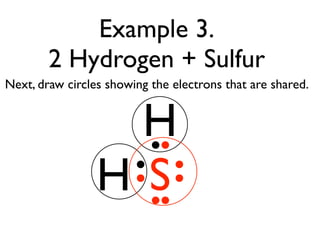
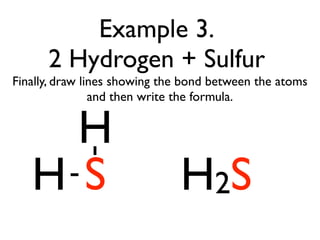
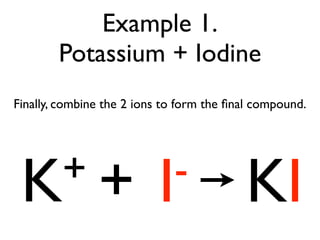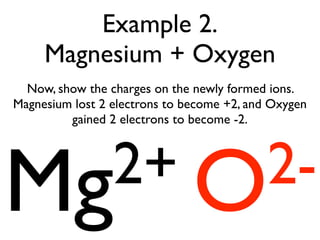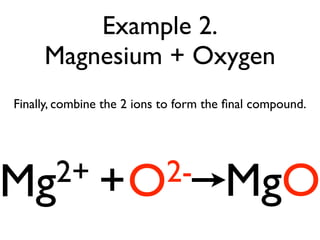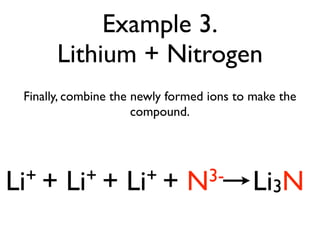This document provides an overview of bonding basics, including ionic and covalent bonds. Ionic bonds form when a metal transfers electrons to a nonmetal, resulting in oppositely charged ions that attract. Covalent bonds form when atoms share electrons to achieve a full outer shell. Examples show Lewis diagrams and representing the transfer or sharing of electrons to form ionic compounds like KI or covalent molecules like H2O.