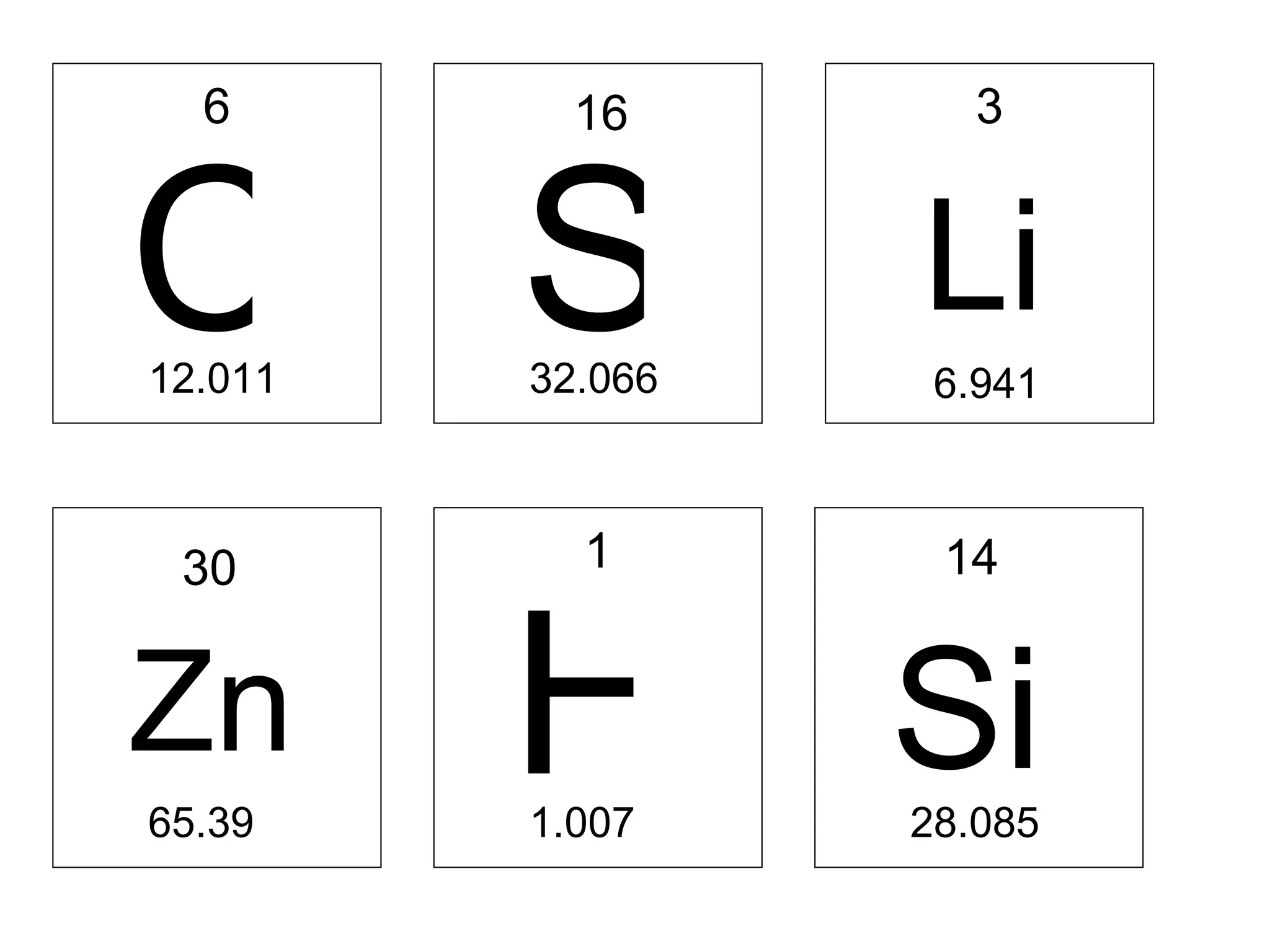- Thompson discovered electrons using cathode ray tubes. Rutherford discovered the nucleus and that it is positively charged through gold foil experiments.
- Chadwick discovered neutrons by observing particles in a beam that were not deflected by a magnetic field.
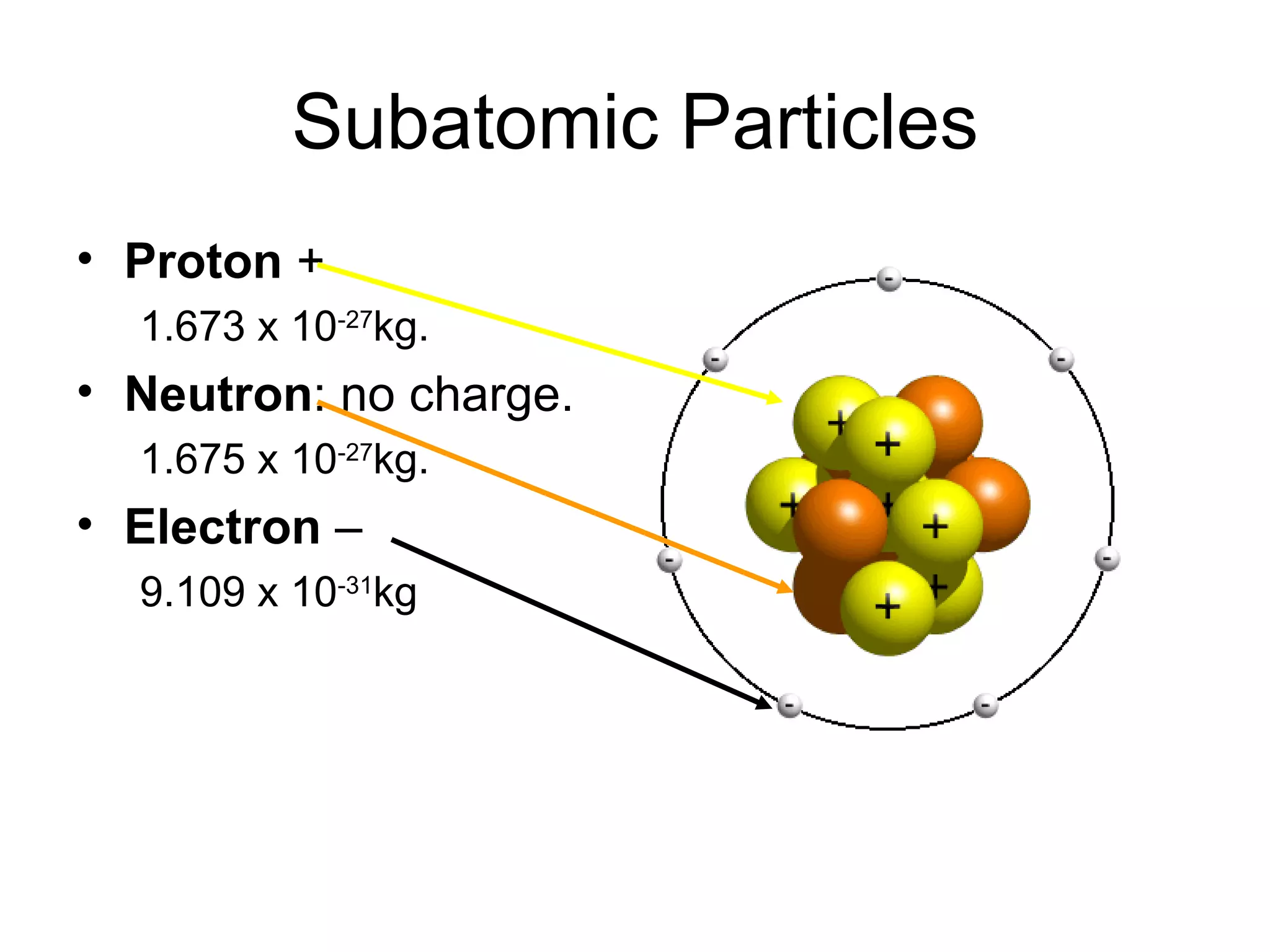
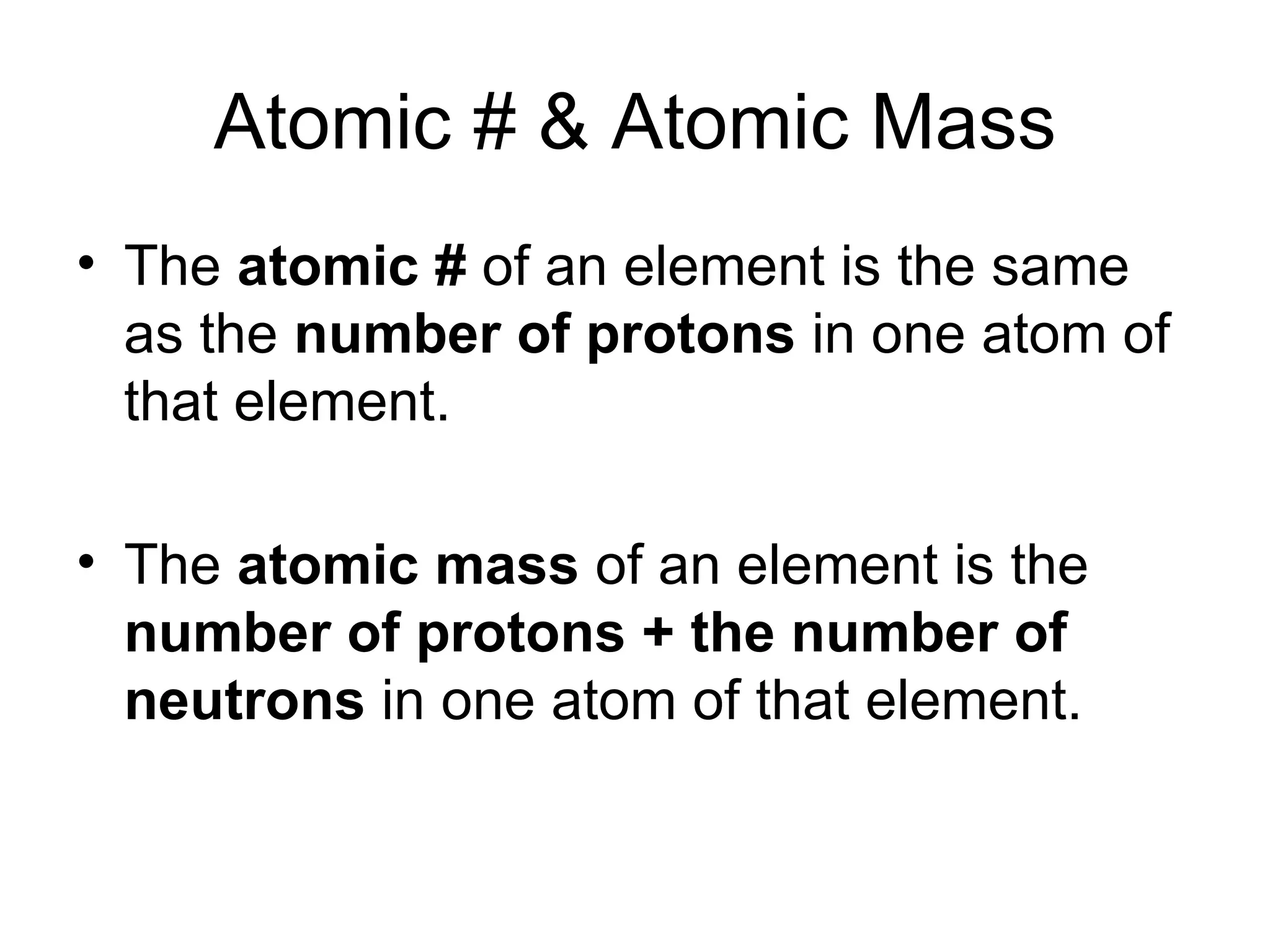
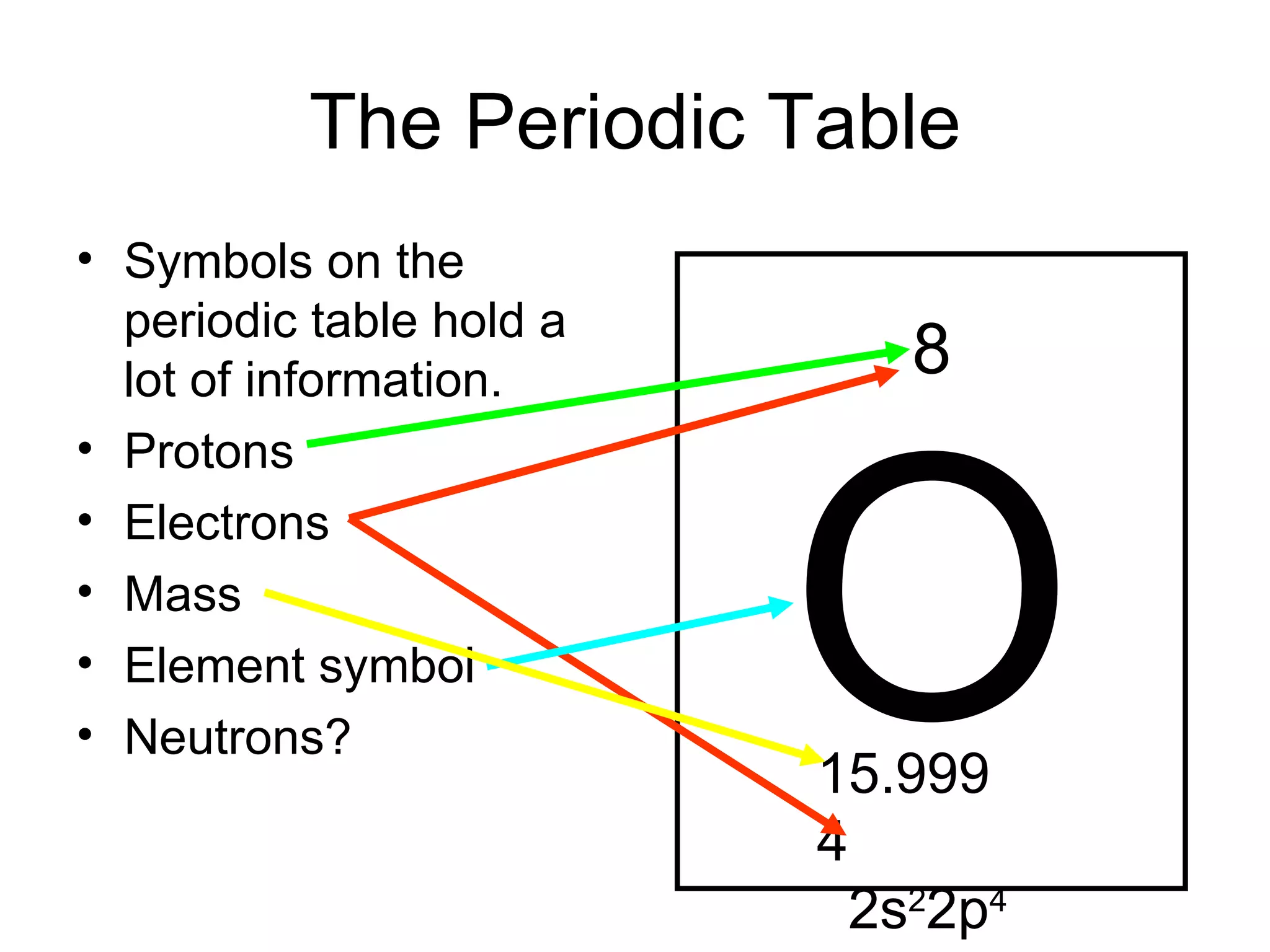
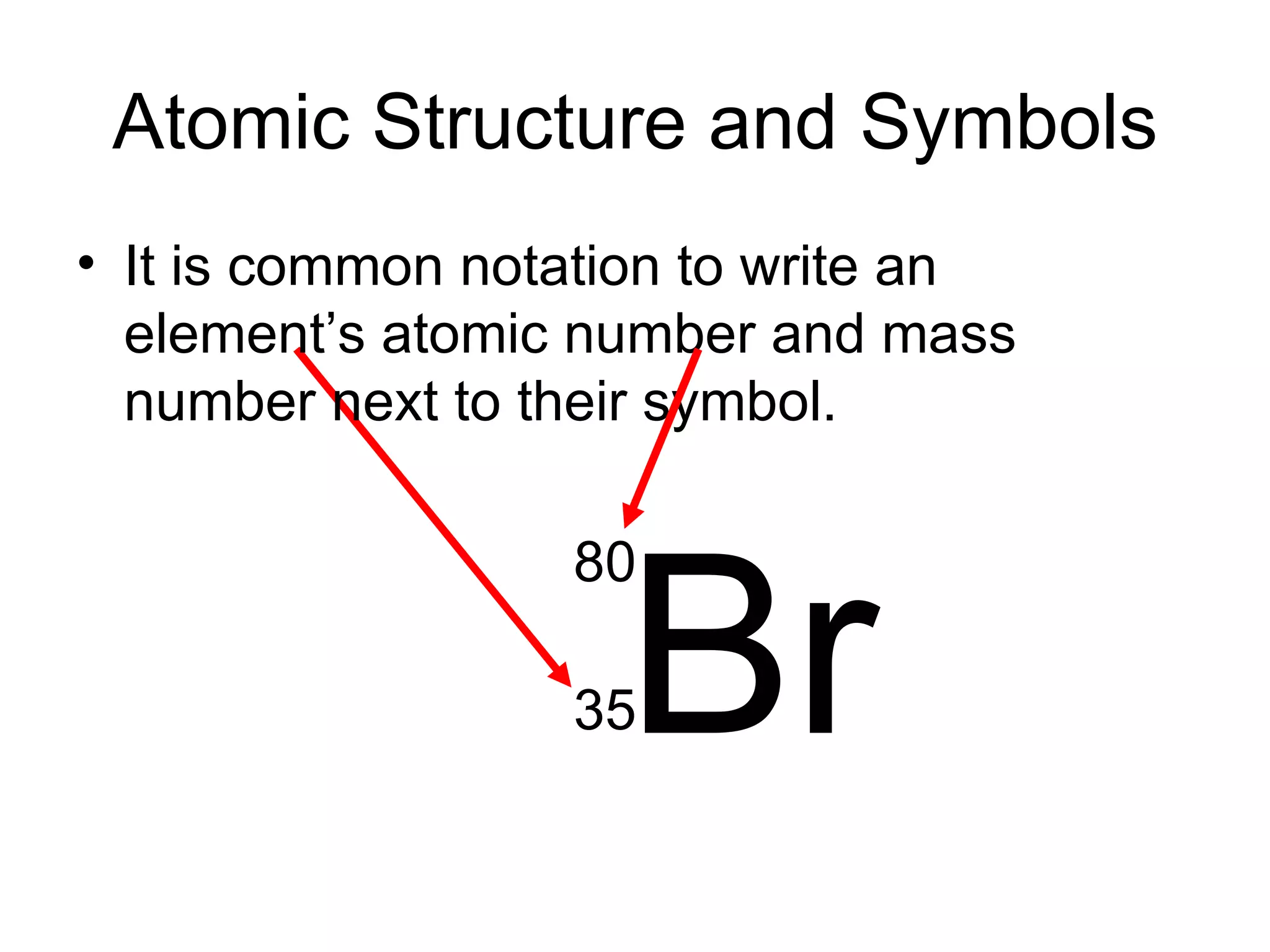
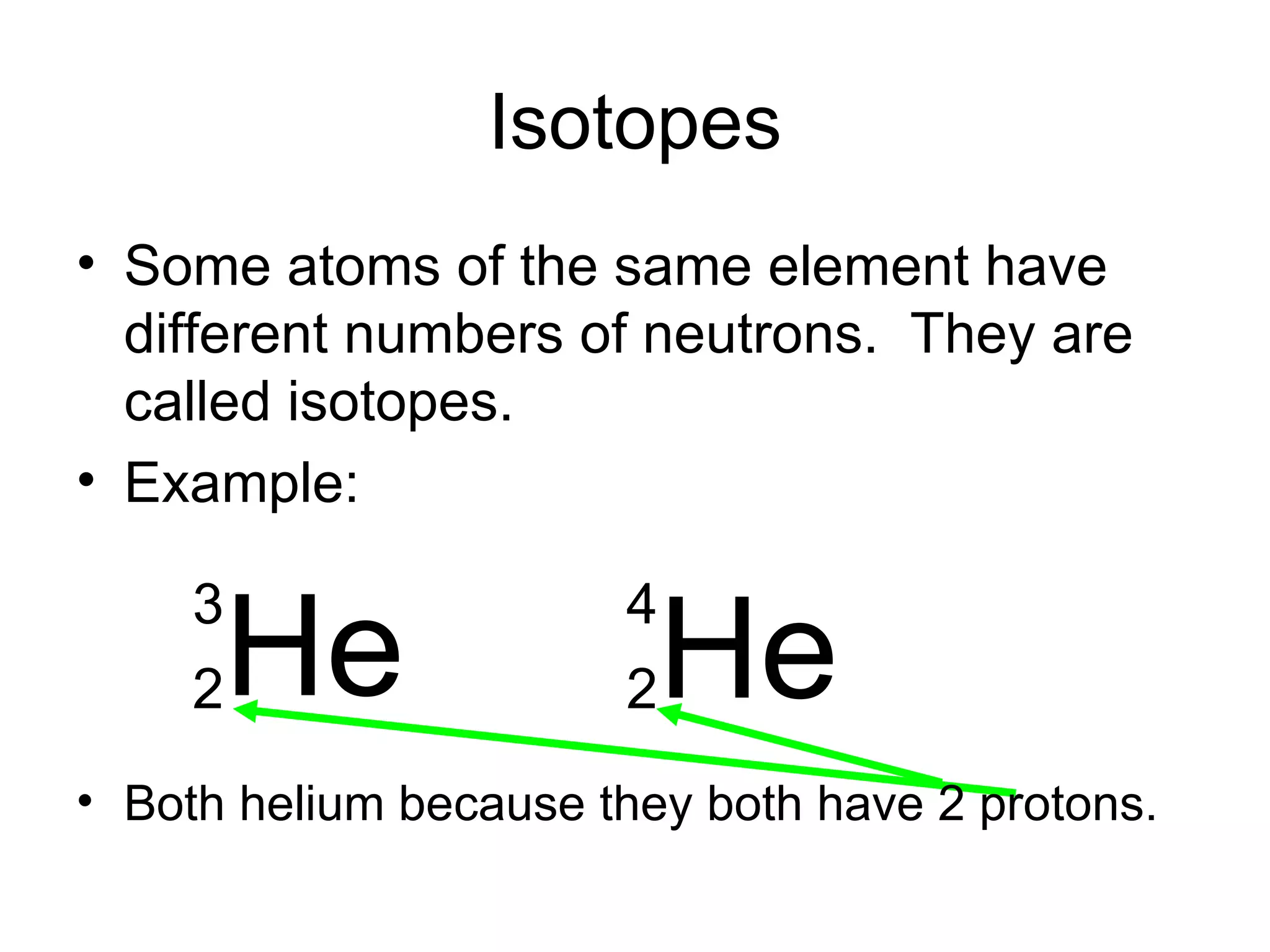
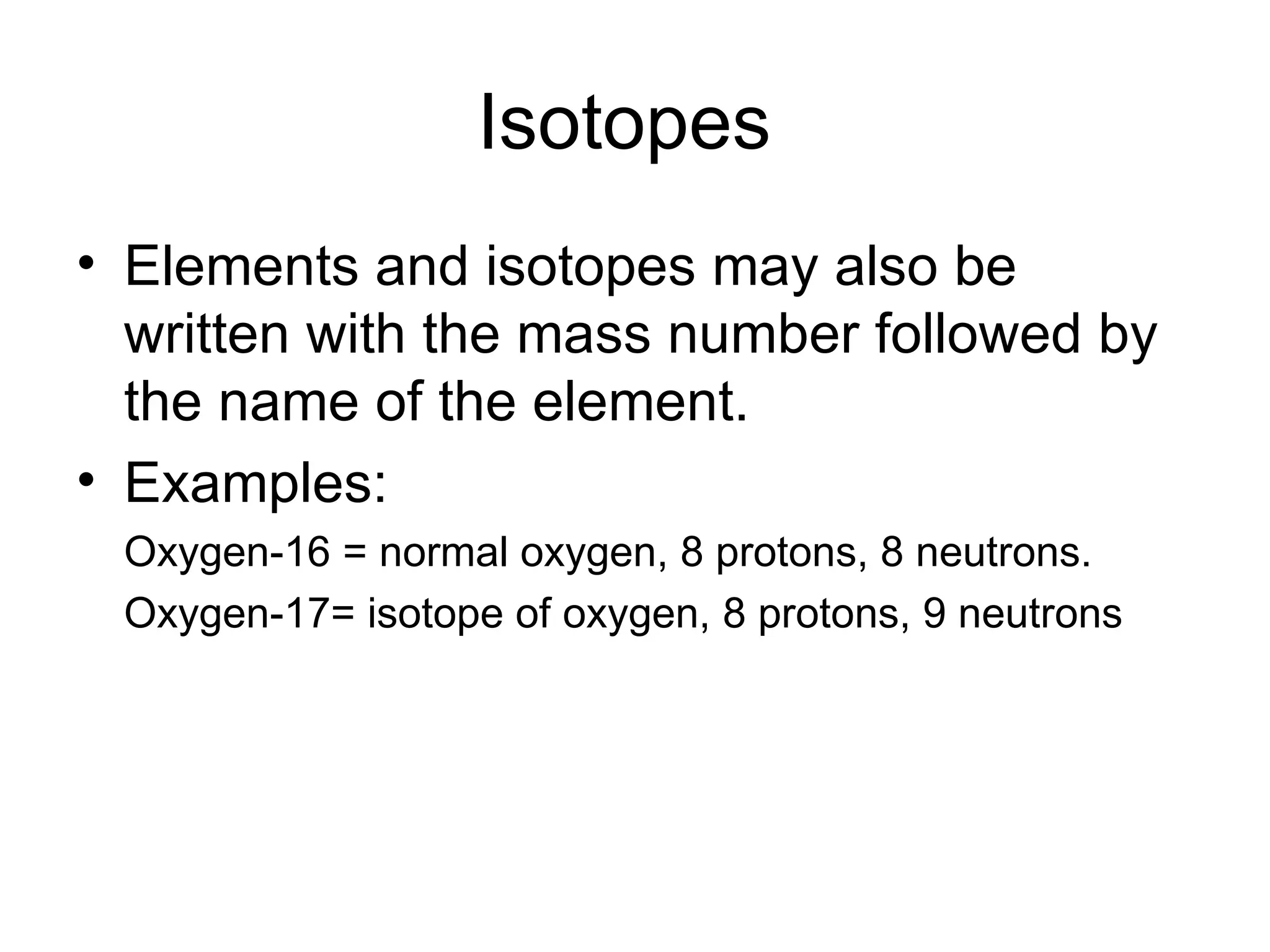
- Atoms are made up of protons, neutrons, and electrons. Protons and neutrons reside in the nucleus, while electrons orbit the nucleus. The number of protons defines the element.