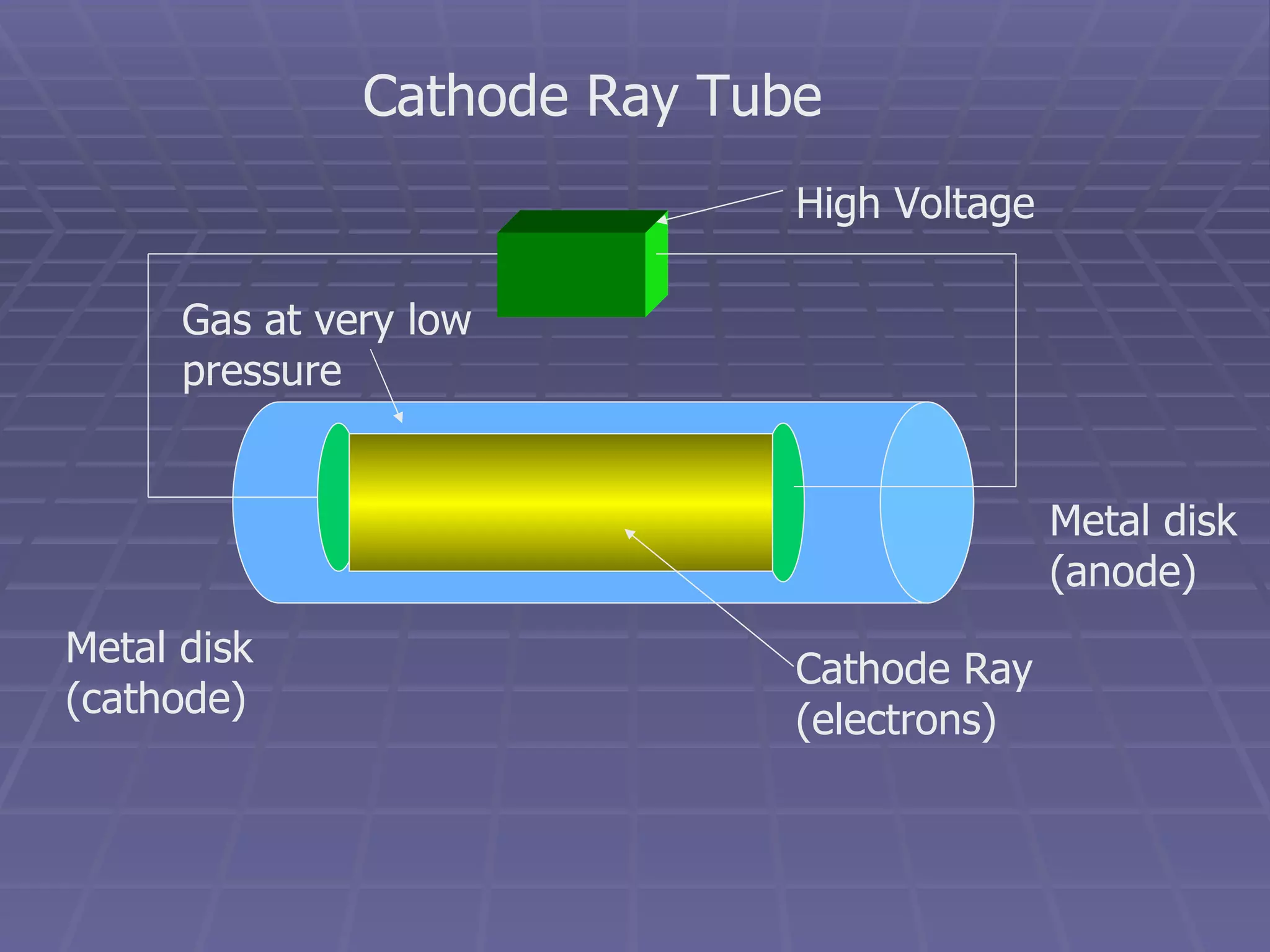
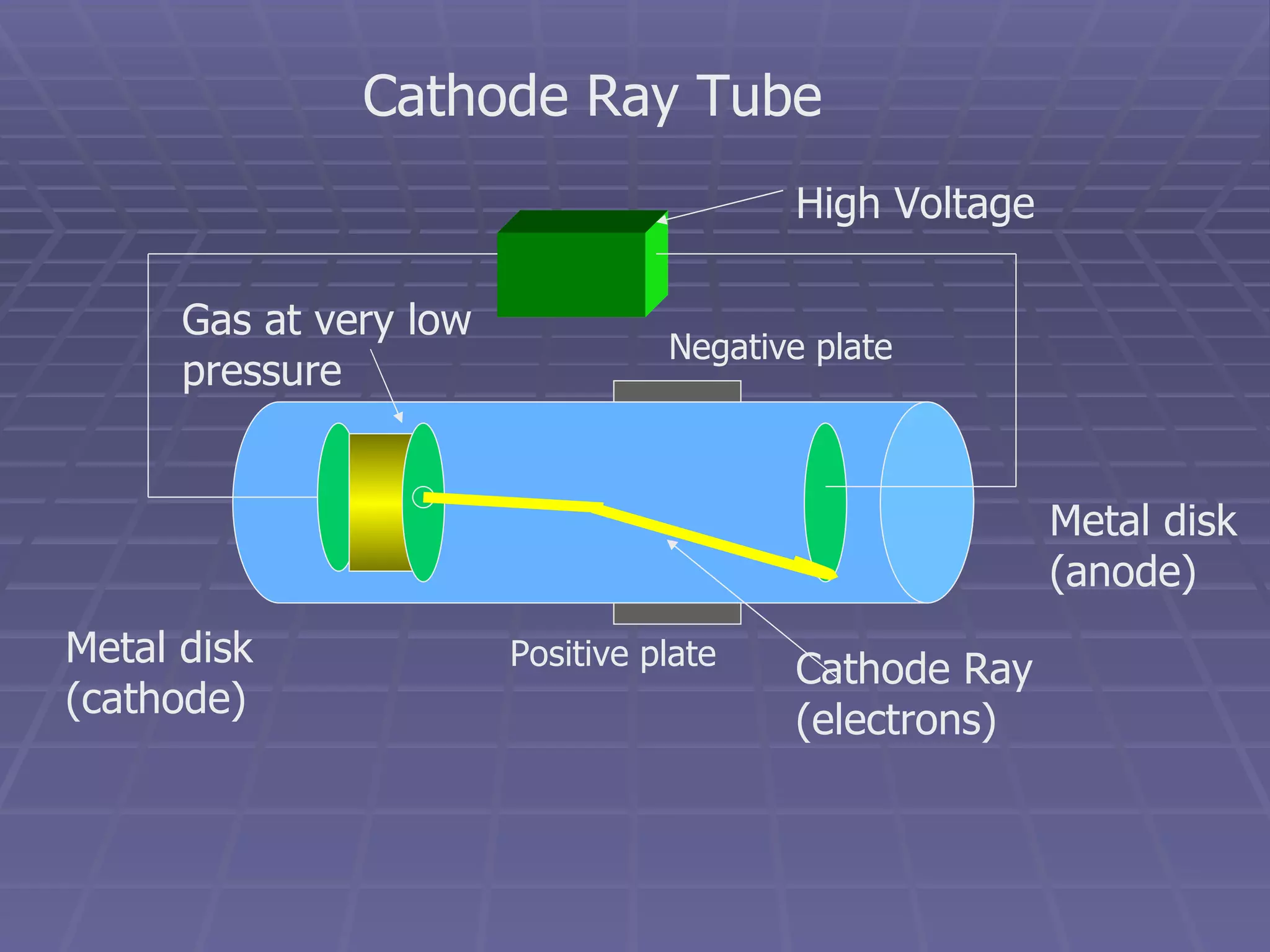
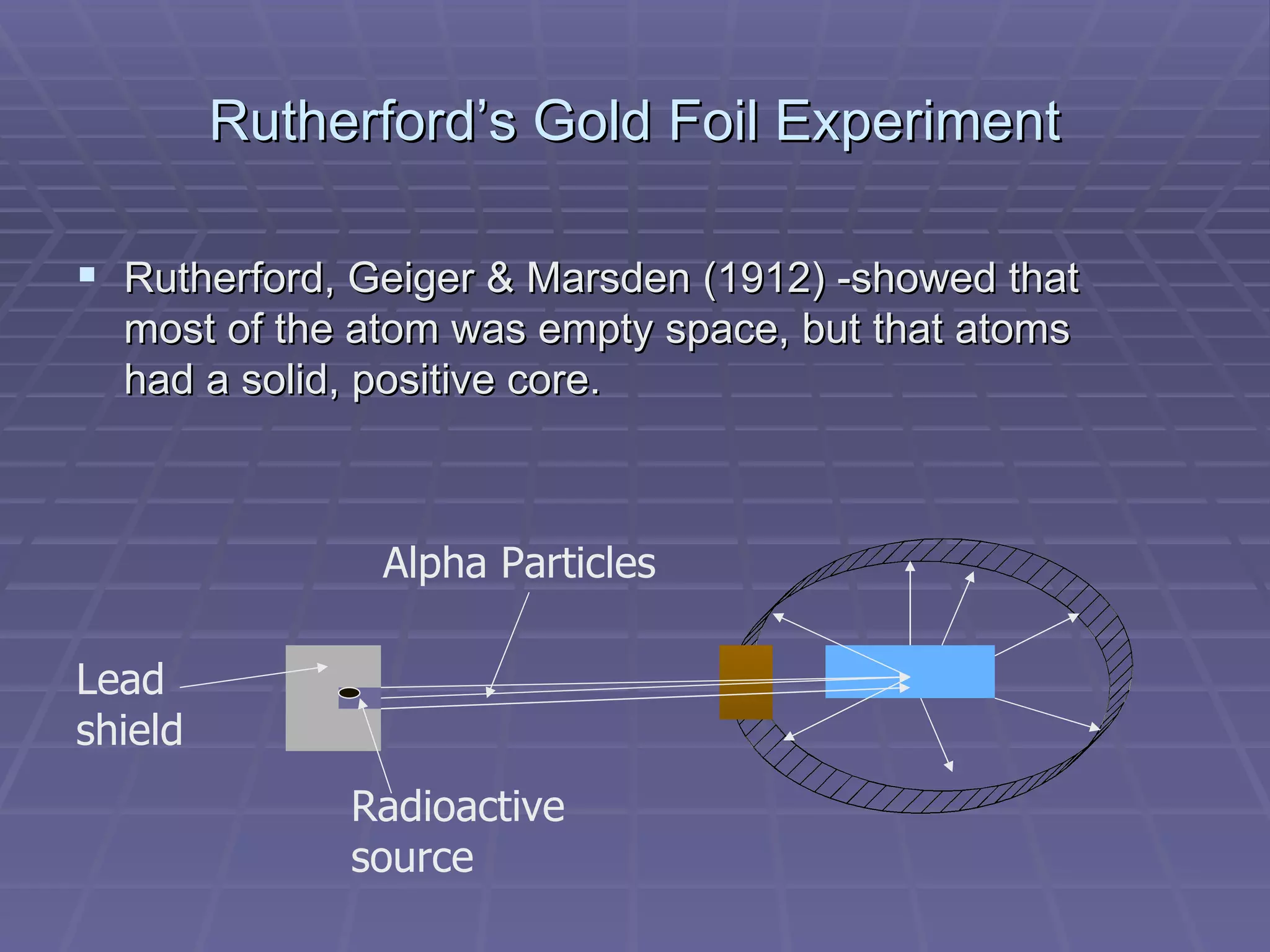
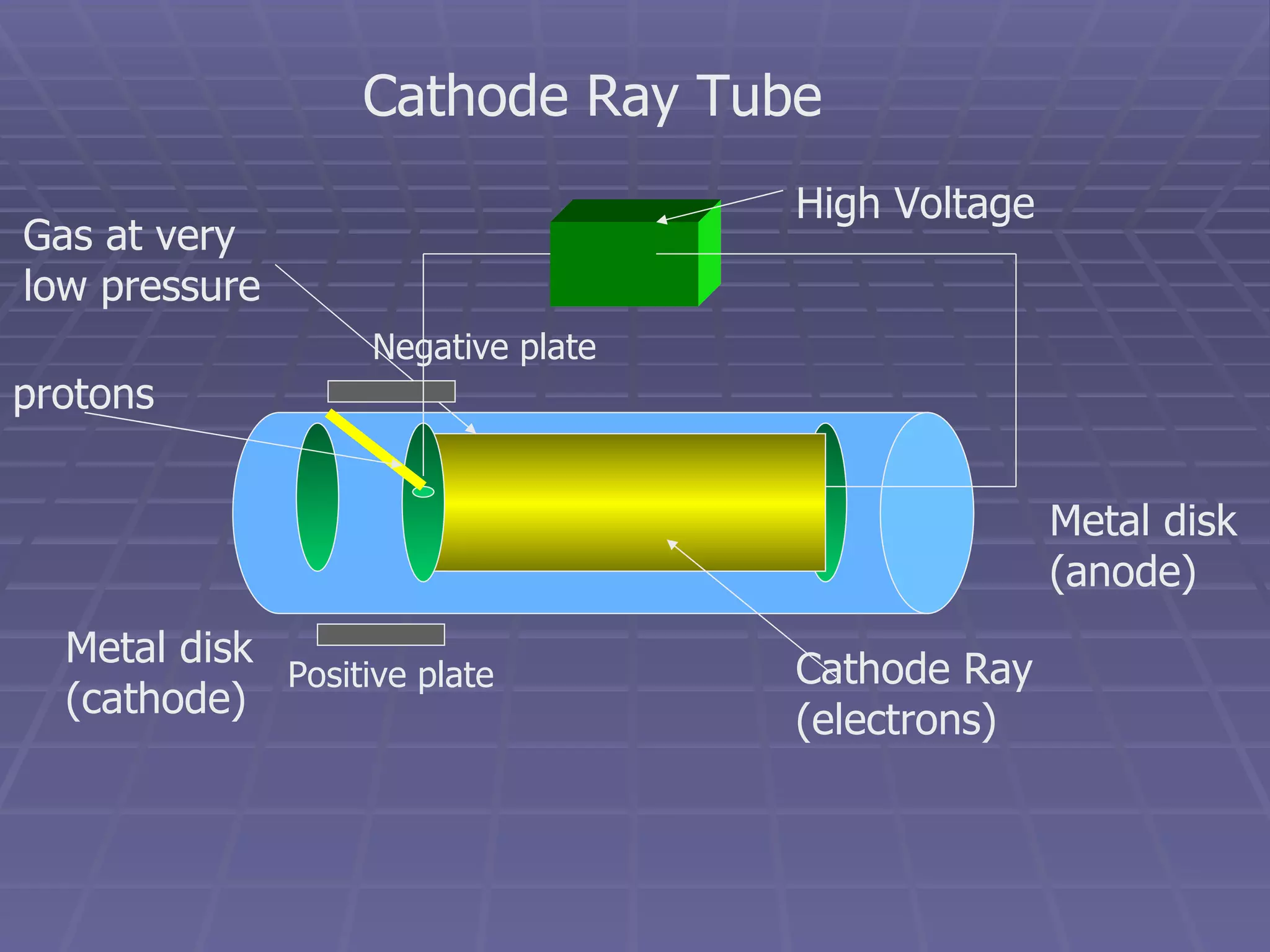
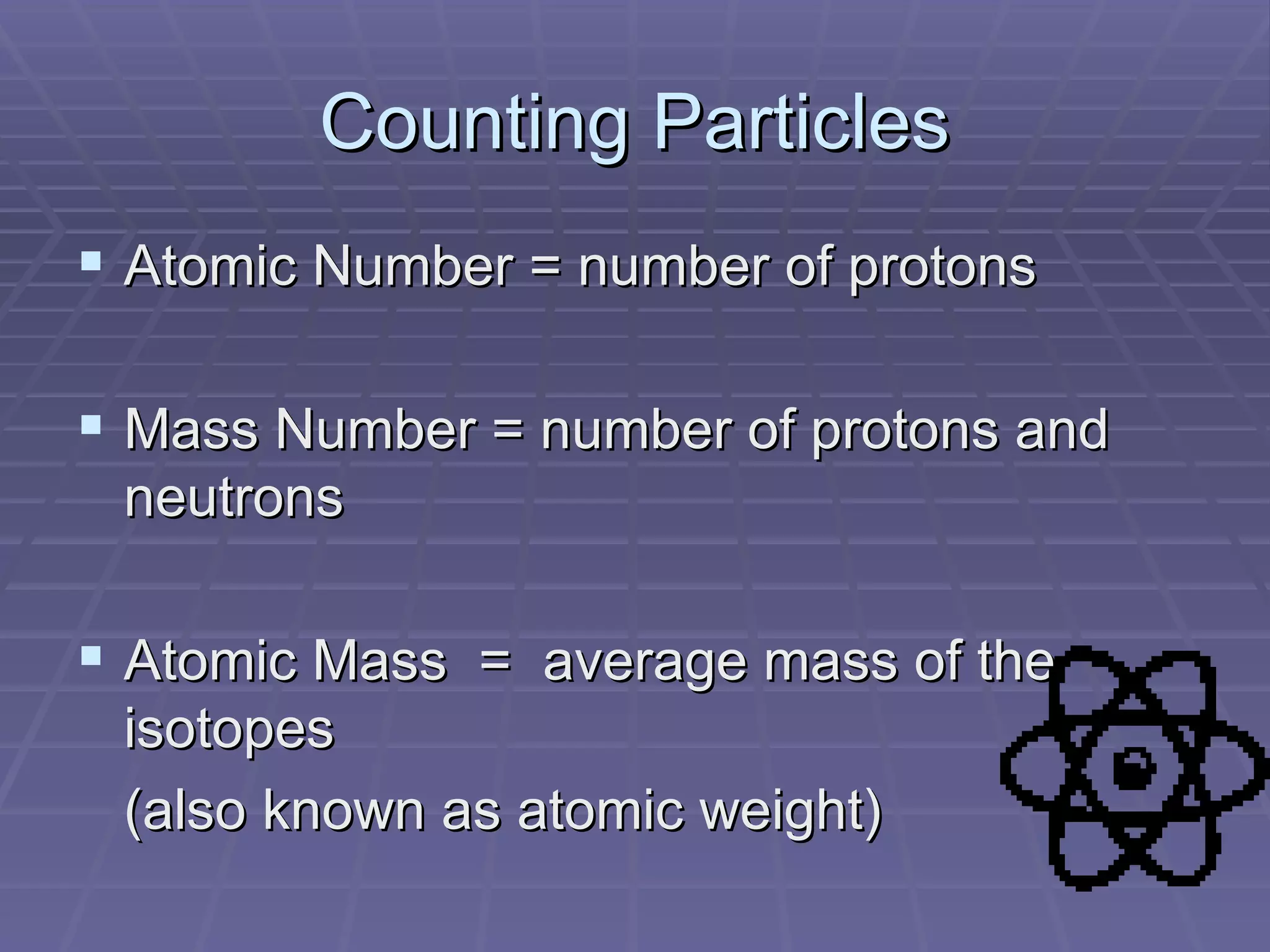
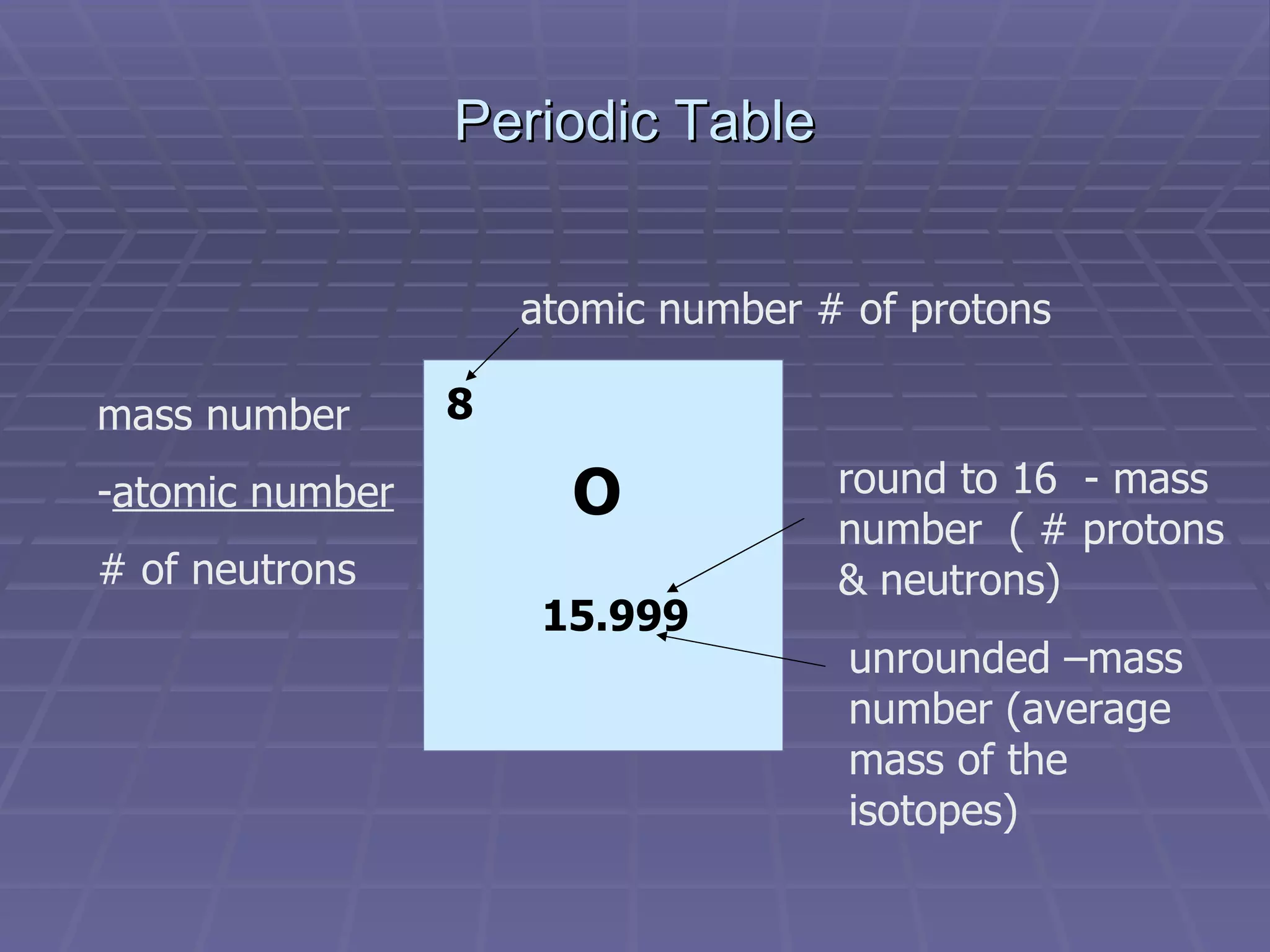
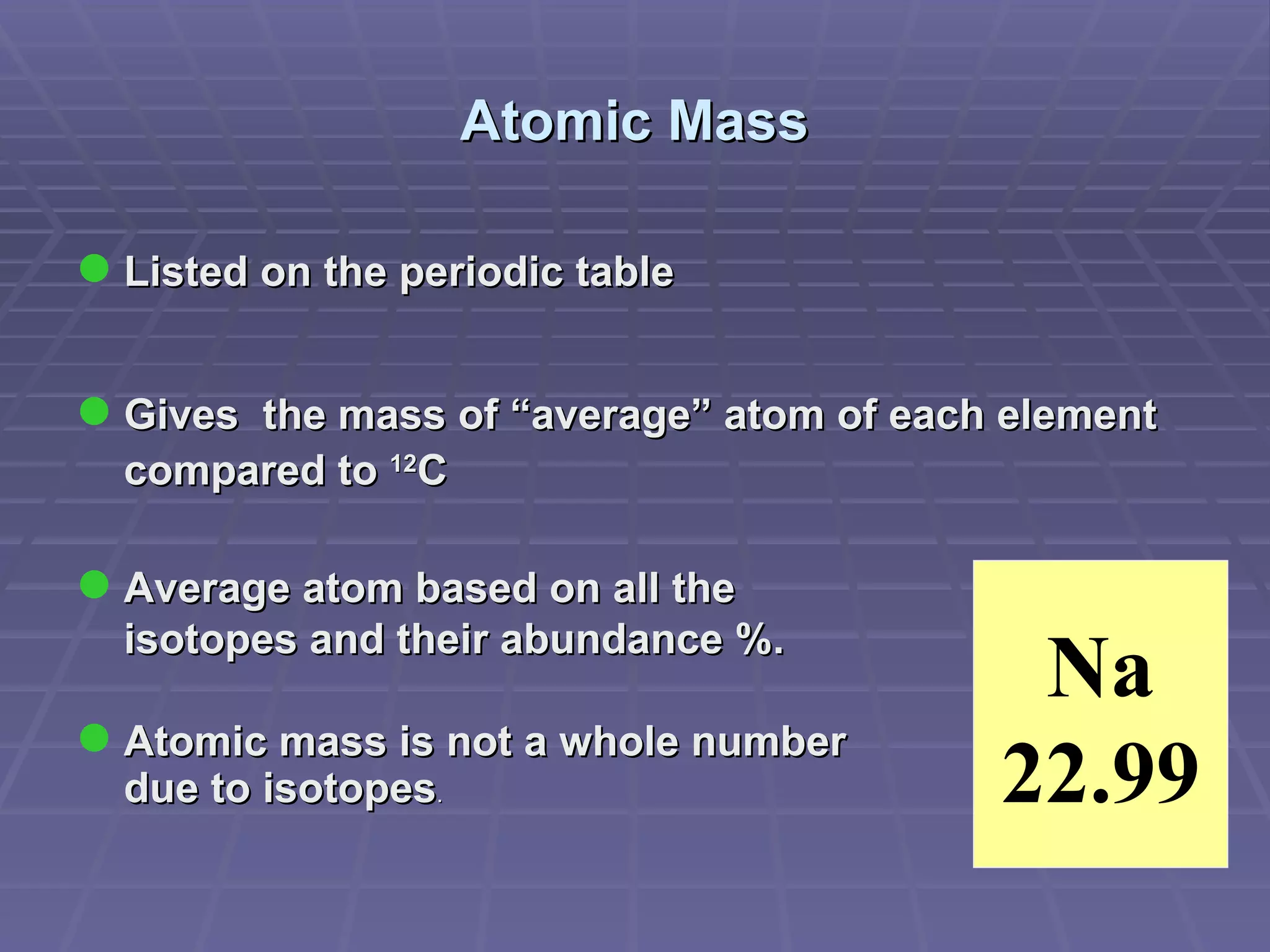
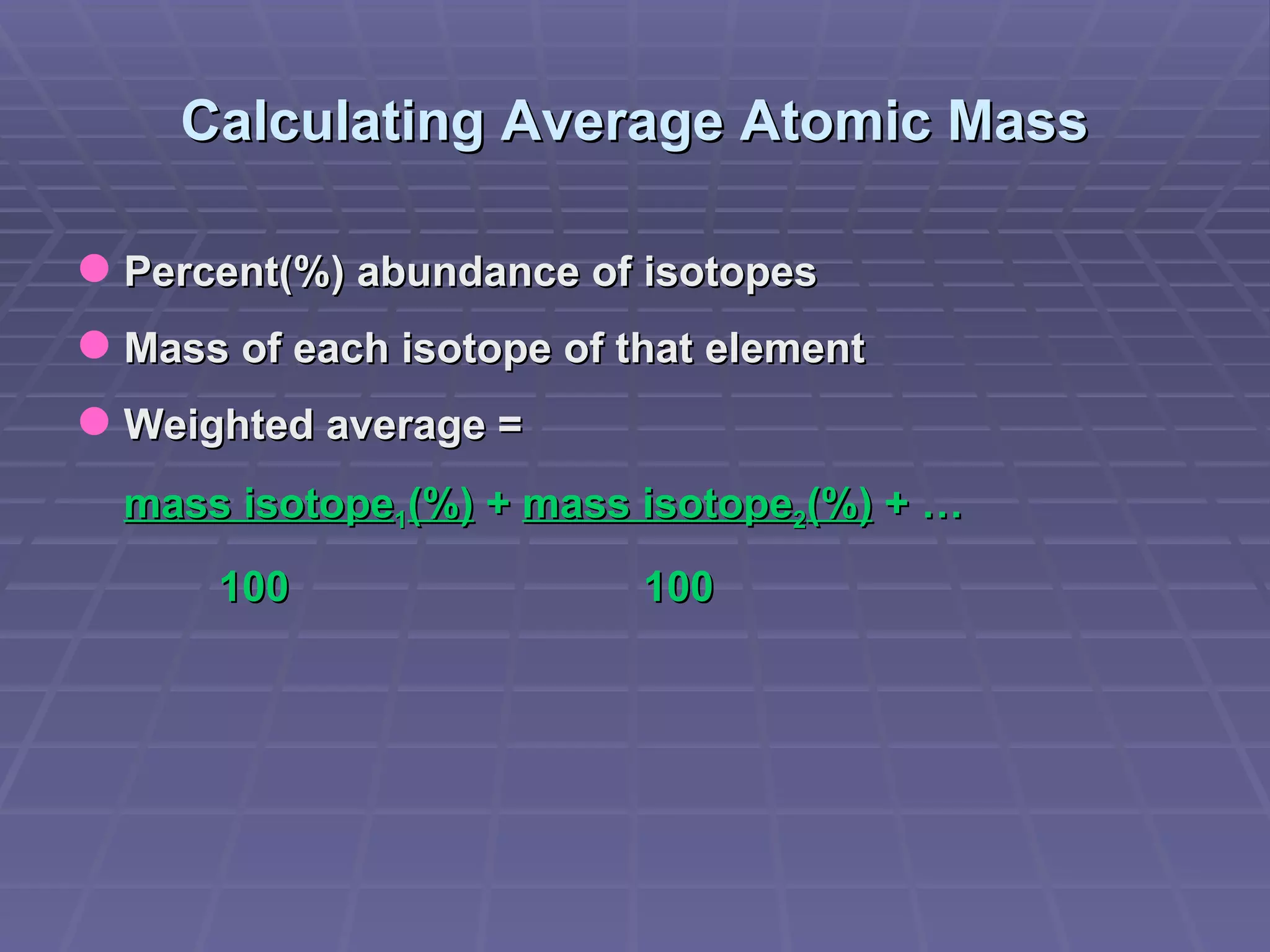
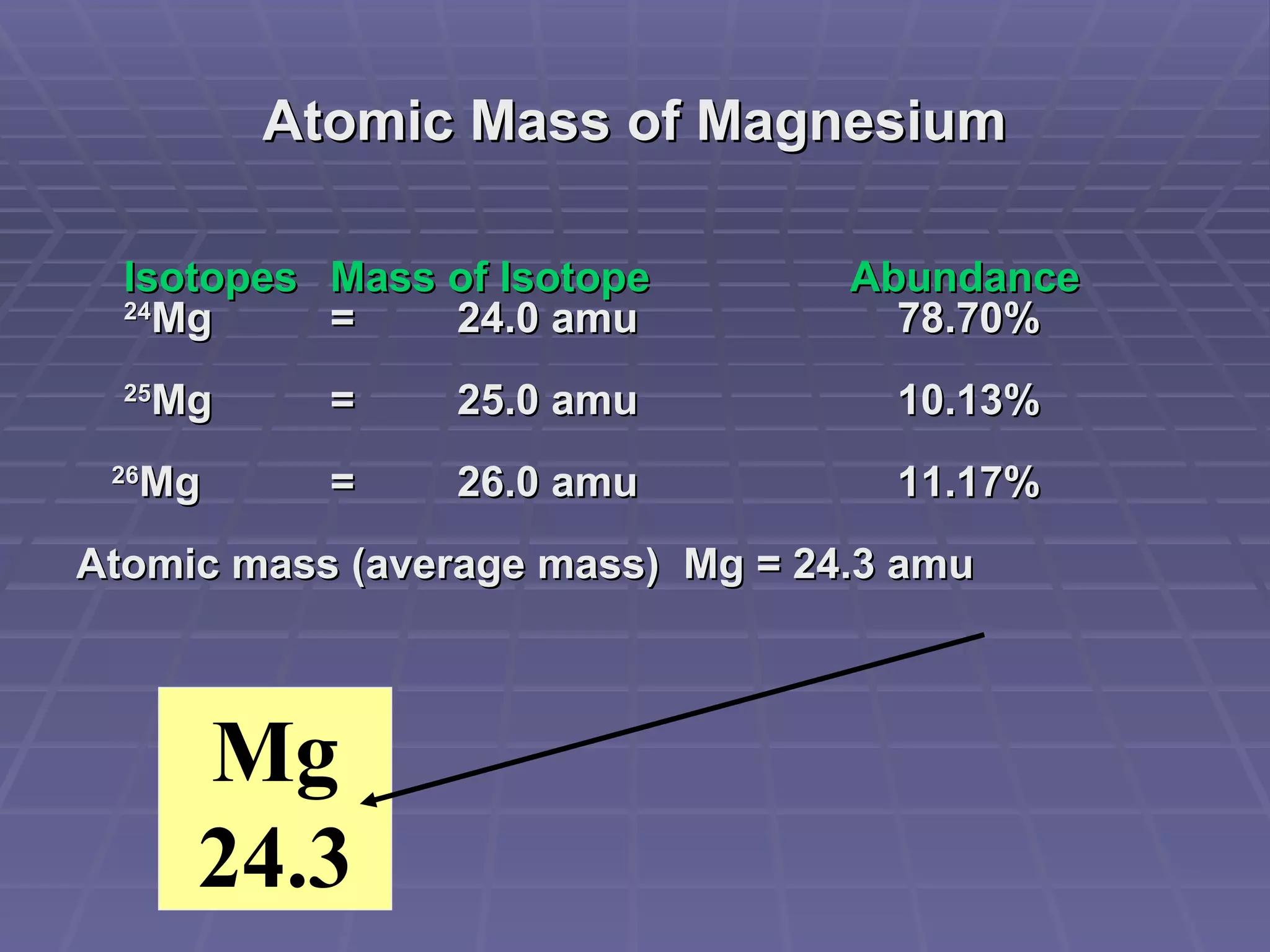
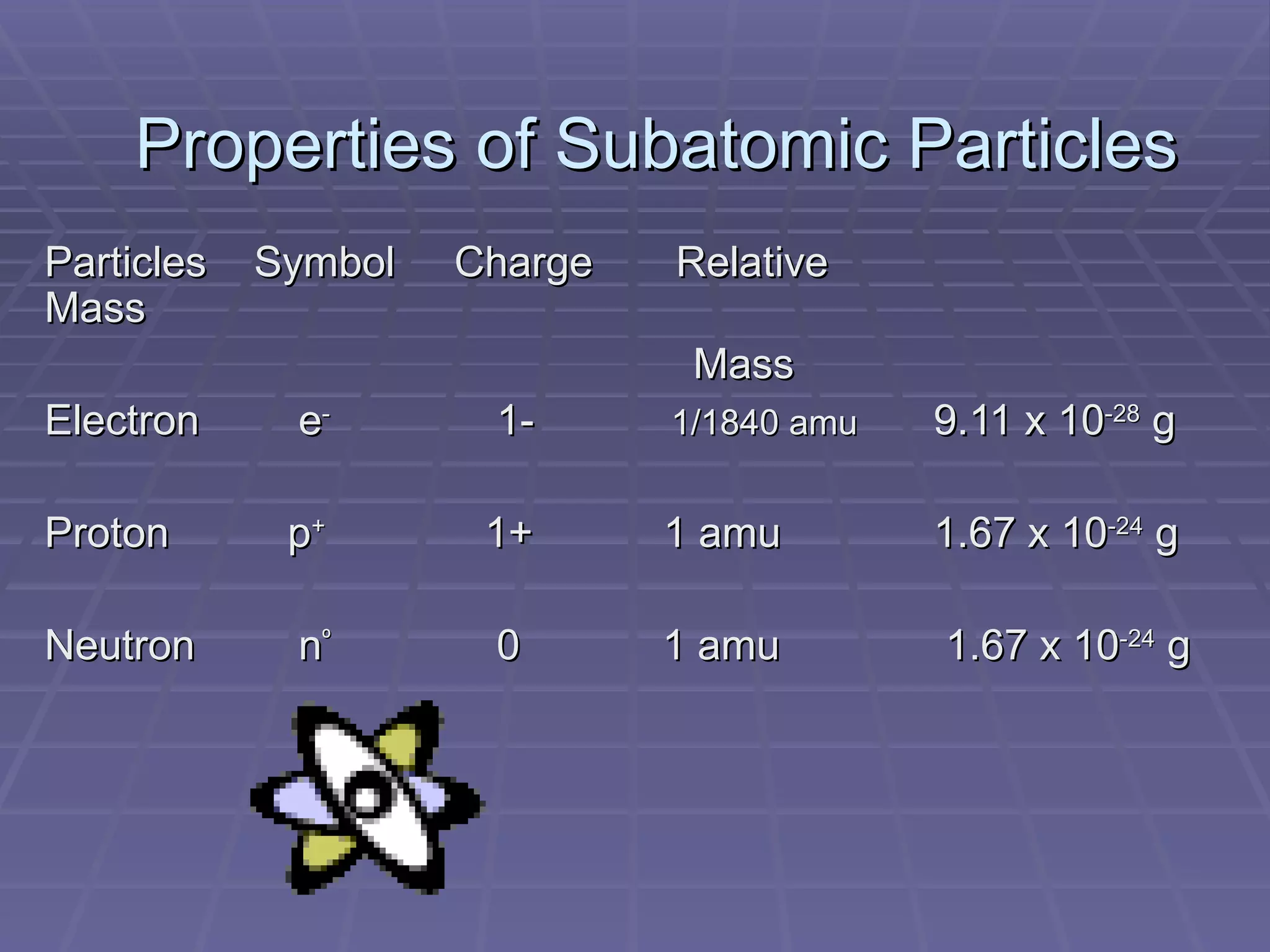
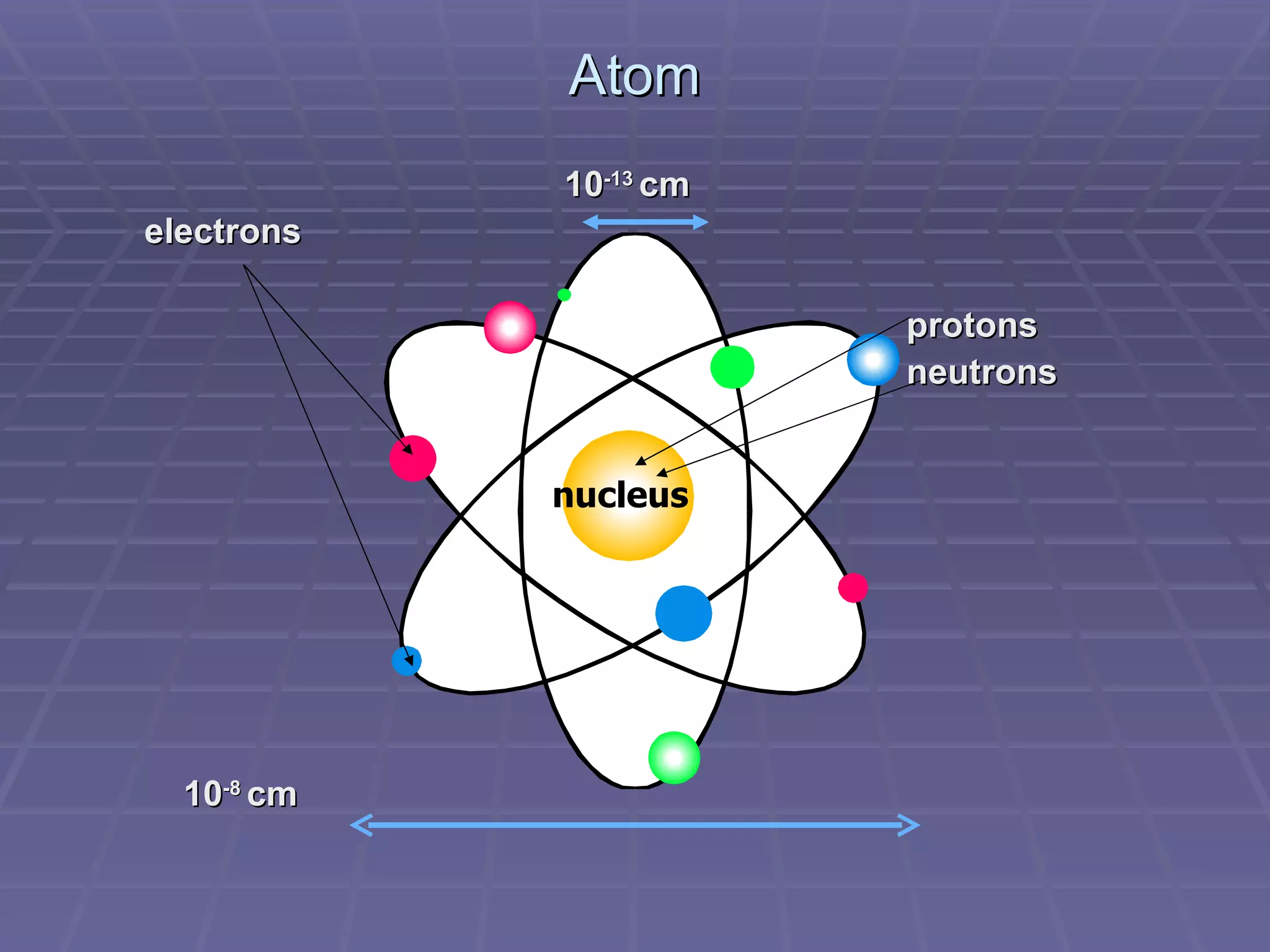
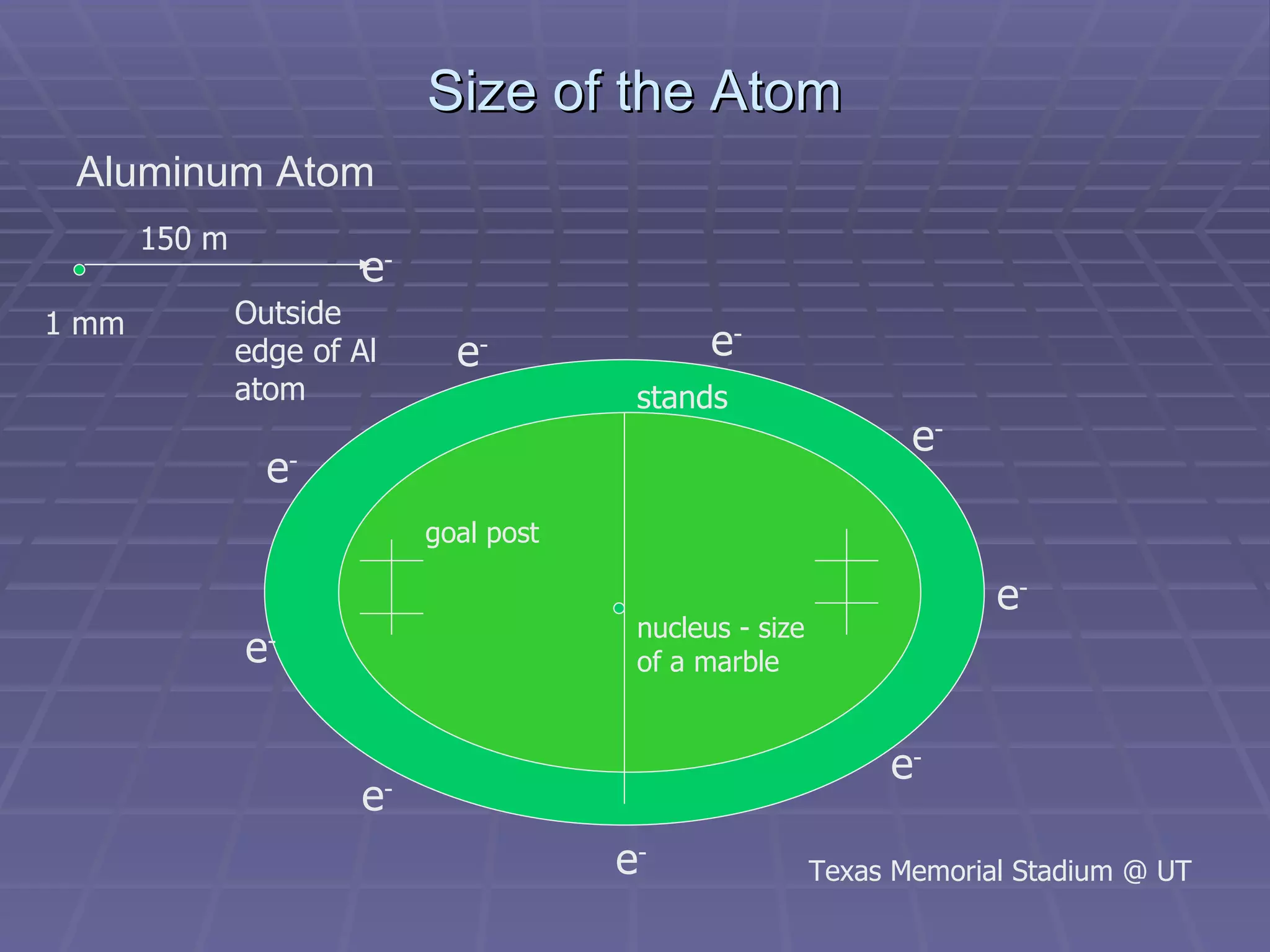
This document provides an overview of the history and development of atomic theory, including key discoveries and models. It describes early ideas from Democritus and Aristotle, foundations laid by laws of conservation of mass, definite proportions, and multiple proportions. Developments include Dalton's atomic theory, discovery of the electron, proton, neutron, isotopes, and the nuclear model of the atom. The periodic table is introduced along with atomic number, mass number, and calculating average atomic mass.