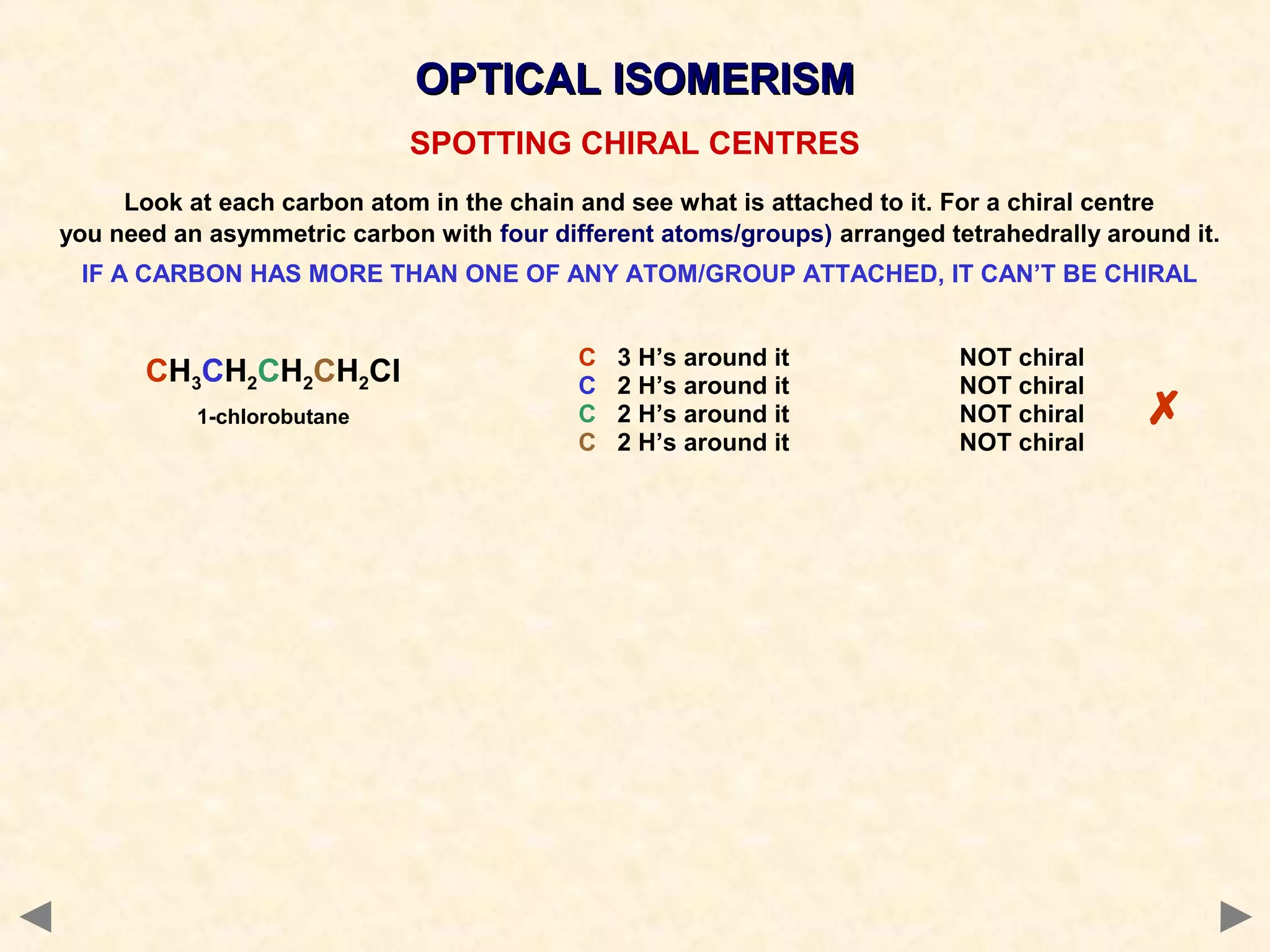
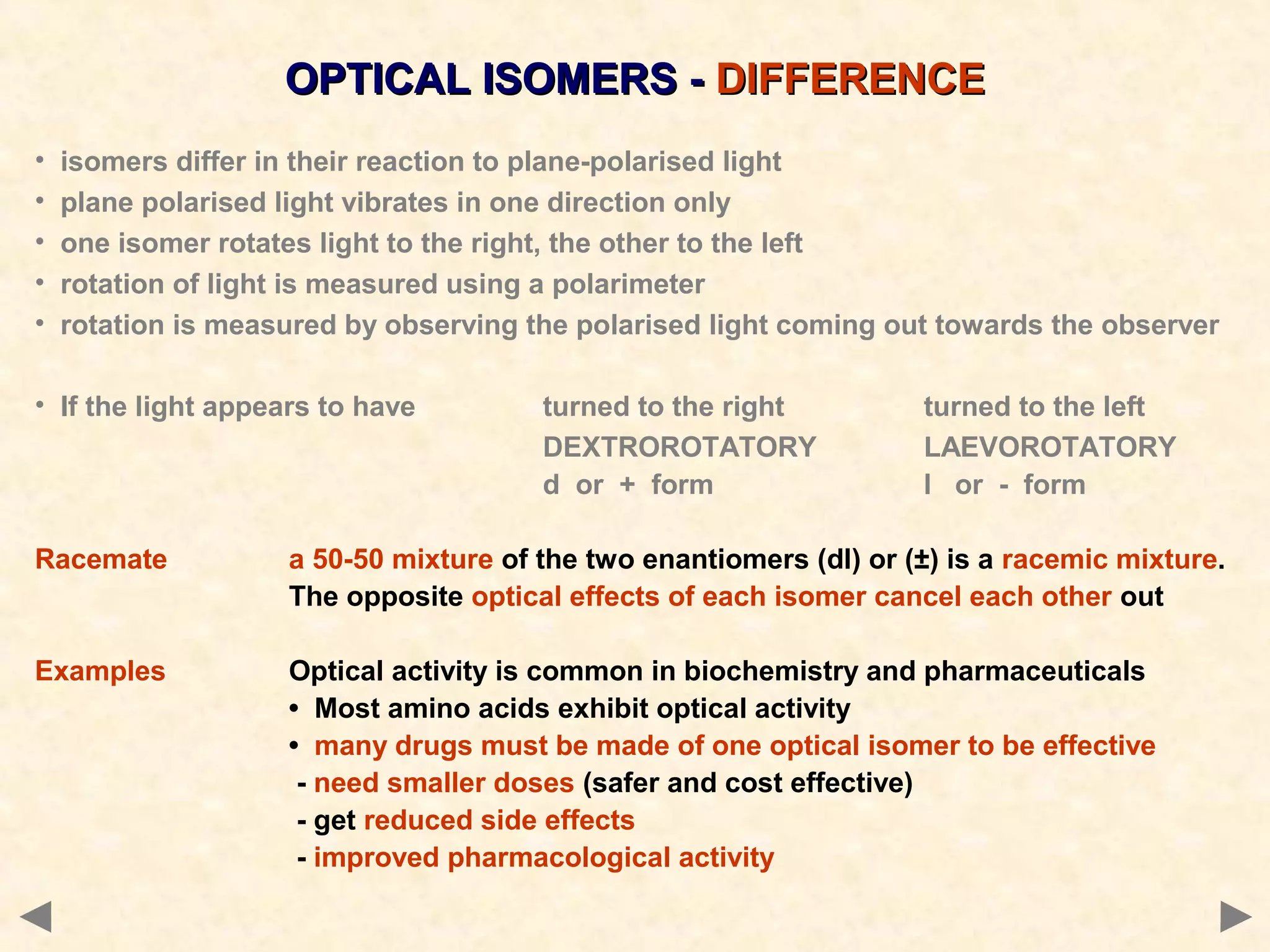
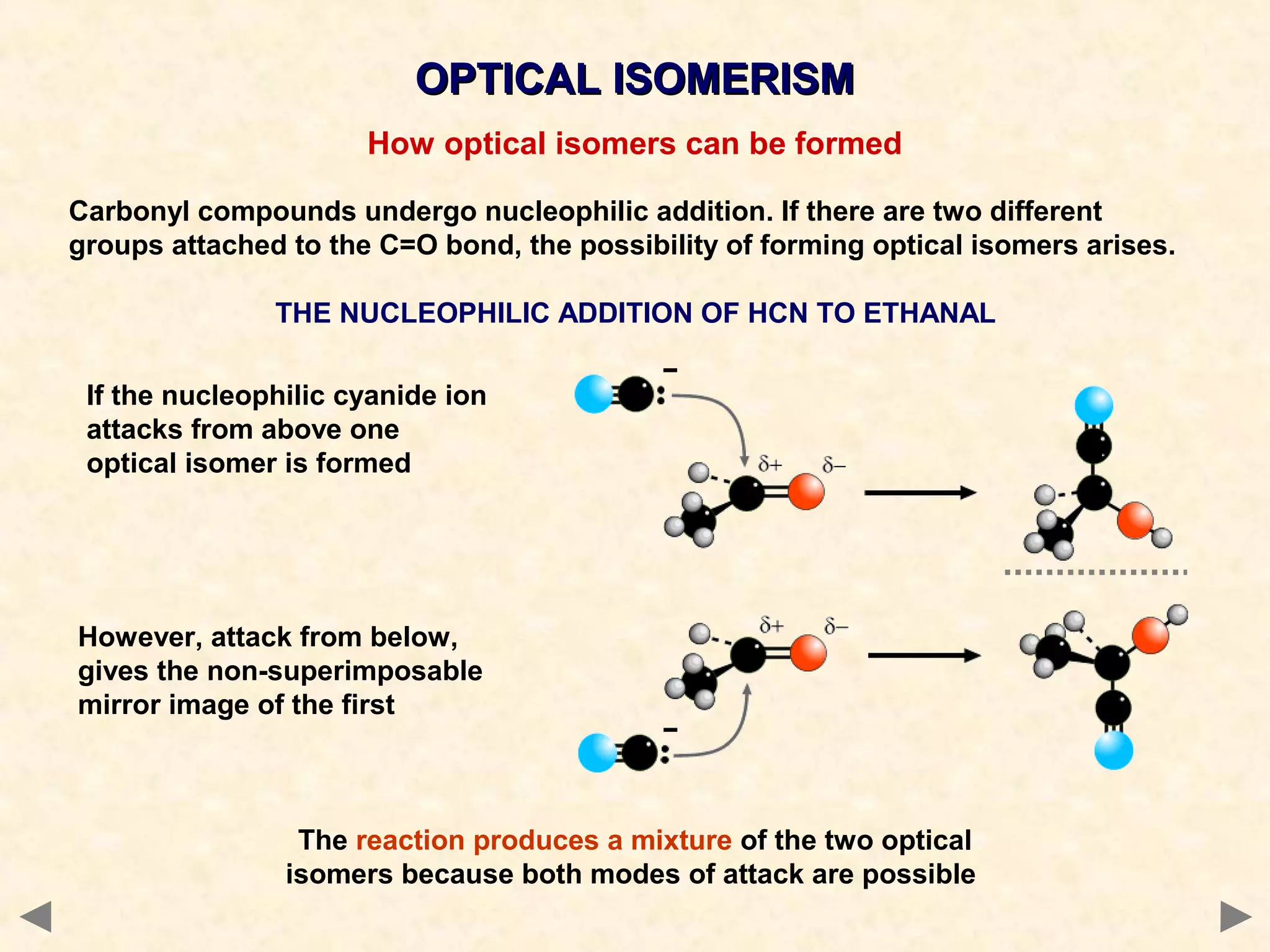
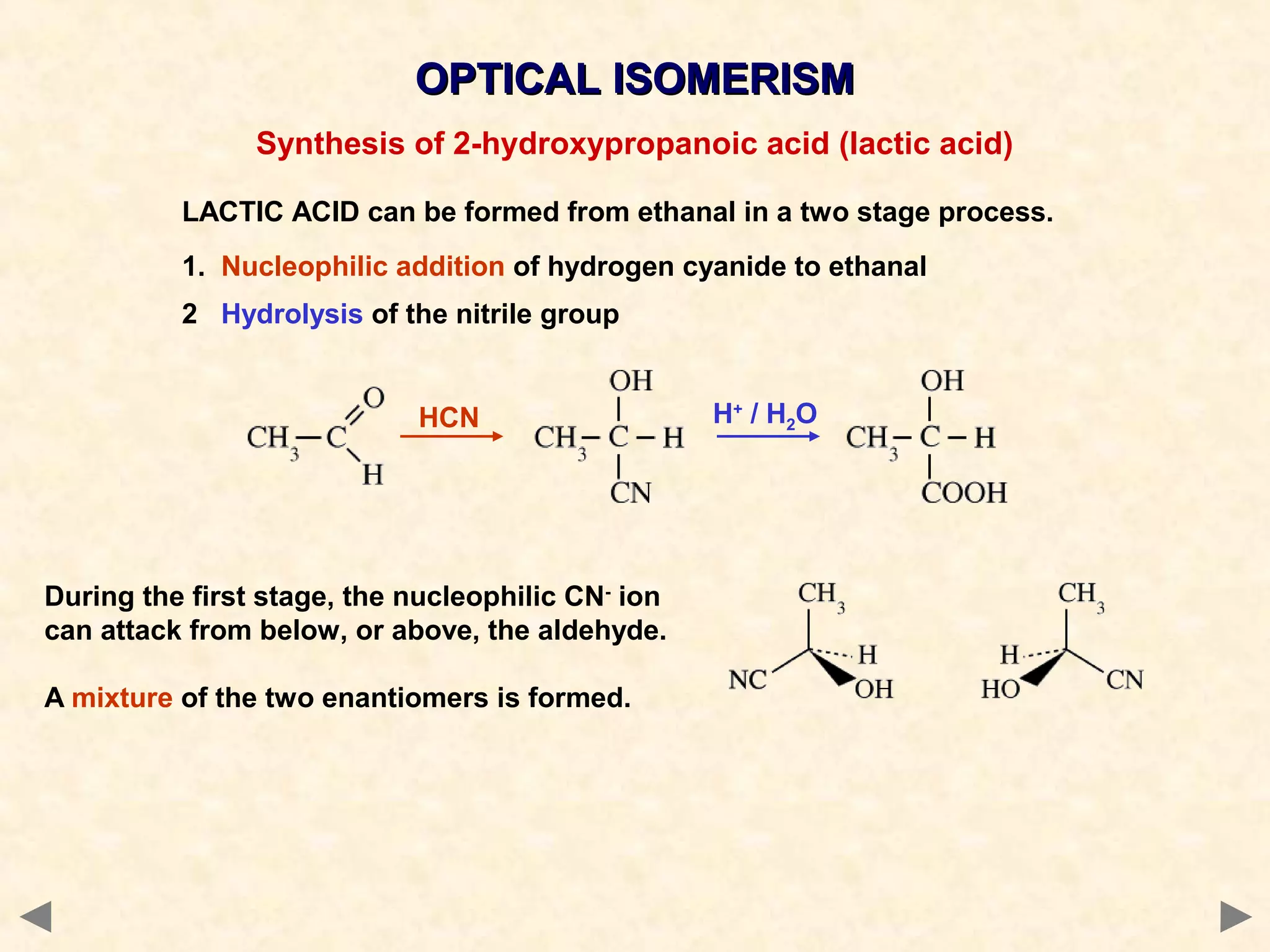
This document discusses optical isomerism, which occurs when chiral molecules have non-superimposable mirror images. Optical isomers, also called enantiomers, differ in how they rotate plane-polarized light and may exhibit different chemical reactivities. The synthesis of lactic acid from aldehyde involves a nucleophilic addition that produces a racemic mixture of both optical isomer forms. The drug thalidomide tragedy demonstrated that only one of a chiral drug's enantiomers may be safe, with unintended consequences if an unseparated racemic mixture is used.