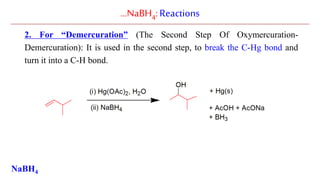
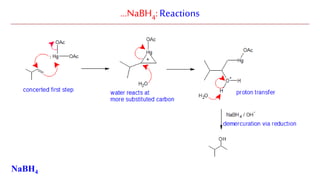
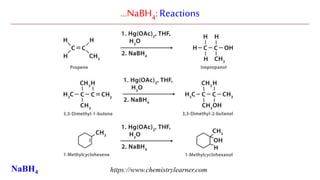
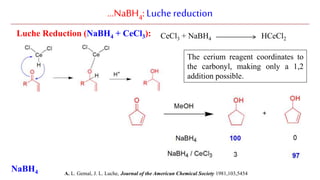
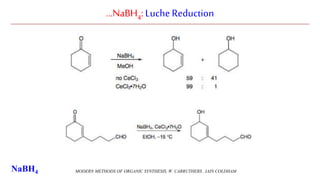
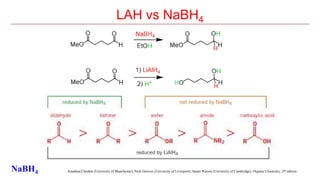
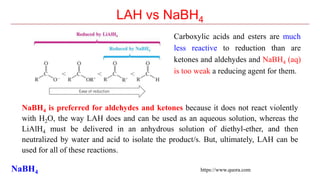
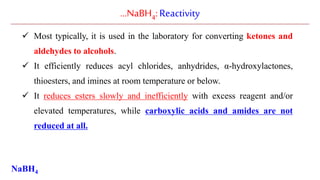
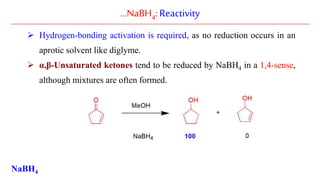
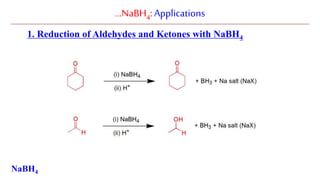
Sodium borohydride is a reducing agent used in organic synthesis. It is commonly used to reduce carbonyl groups such as aldehydes and ketones to alcohols. The reduction occurs via a two-step mechanism where the borohydride first adds to the carbonyl carbon, then a proton transfers in a second step. Sodium borohydride is a mild reducing agent and selectively reduces carbonyls over other functional groups. It is preferred over lithium aluminum hydride for carbonyl reductions due to its milder and more controlled reactivity in aqueous conditions.

![…NaBH4:Structure
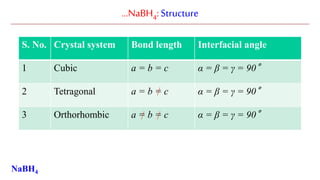
NaBH4 is a salt, consisting of the tetrahedral [BH4]− anion.
The solid is known to exist as three polymorphs: α, β and γ.
The stable phase at room temperature and pressure is α-NaBH4, which is
cubic and adopts an NaCl-type structure.
At a pressure of 6.3 GPa, the structure changes to the tetragonal β-NaBH4
and at 8.9 GPa, the orthorhombic γ-NaBH4 becomes the most stable.
NaBH4
www.wikipedia.com](https://image.slidesharecdn.com/3-200907132613/85/3-NaBH4-3-320.jpg)

![…NaBH4:Mechanism(2 steps)
i. In the first step, H- detaches from the BH4
– and adds to the carbonyl carbon
([1,2]-addition). This forms the C-H bond, and breaks the C-O bond, resulting in
a new lone pair on the oxygen, which makes the oxygen negatively charged
(alkoxides, as they are deprotonated alcohols).
ii. In the second step, a proton from water (or an acid) is added to the alkoxide to
make the alcohol. This is performed at the end of the reaction, a step referred to
as the workup.
NaBH4](https://image.slidesharecdn.com/3-200907132613/85/3-NaBH4-9-320.jpg)