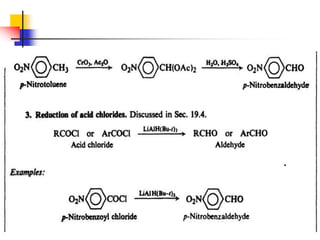
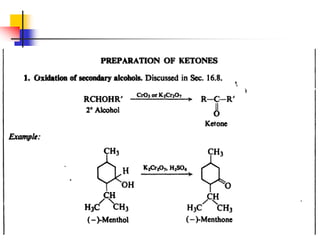
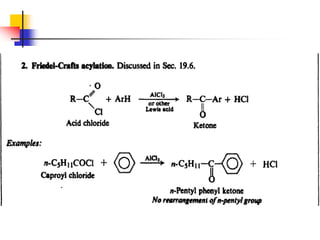
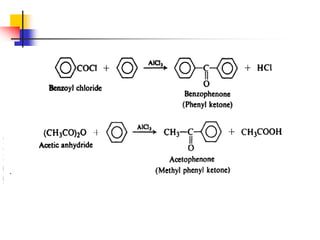
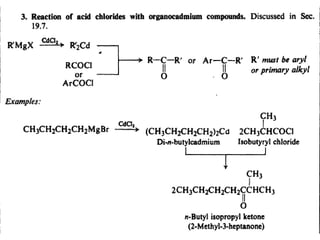
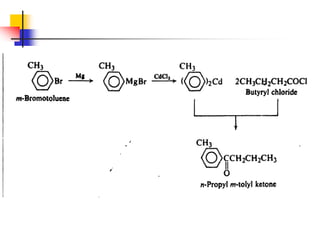
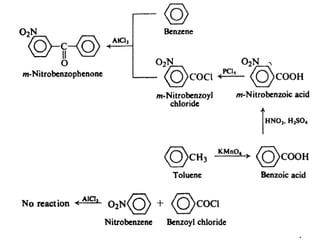
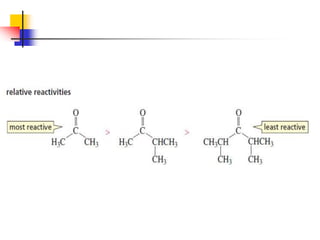
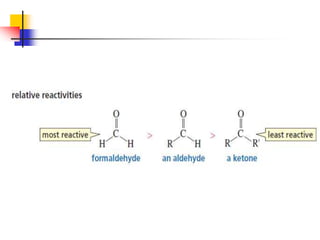
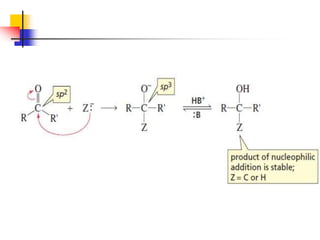
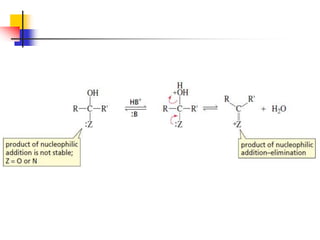
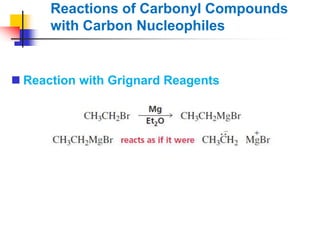
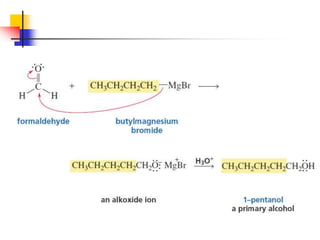
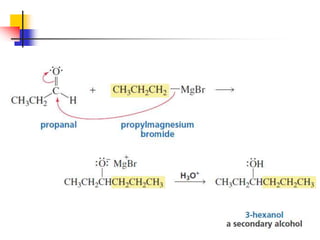
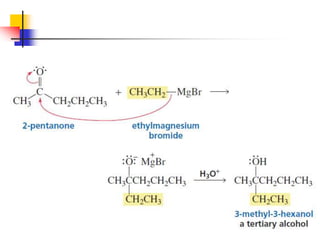
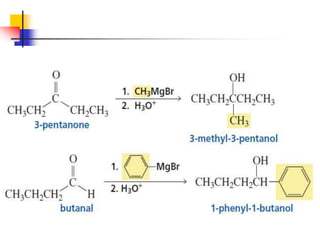
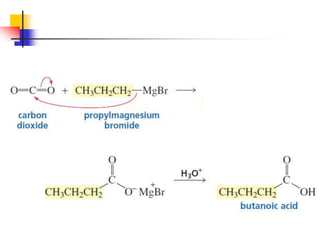
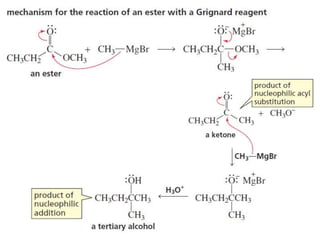
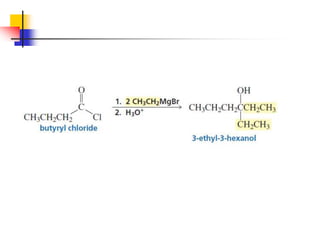
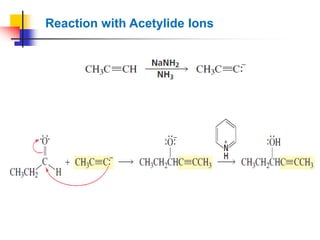
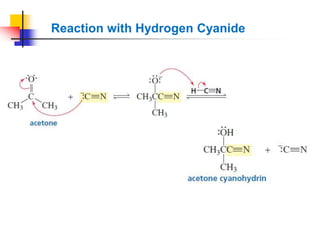
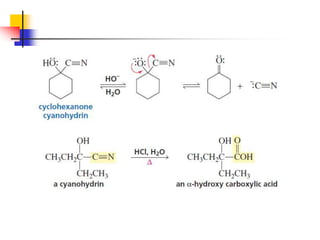
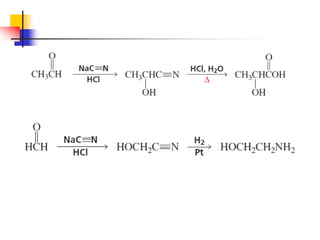
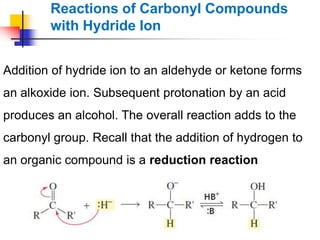
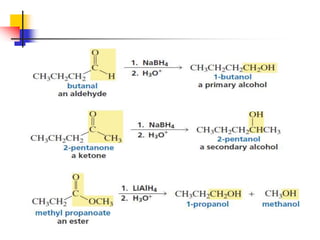
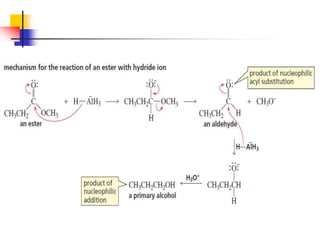
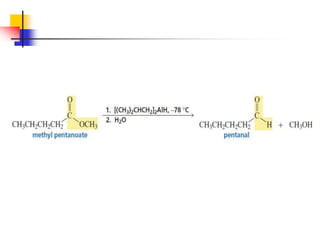
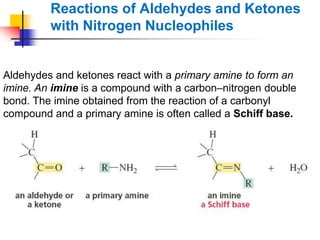
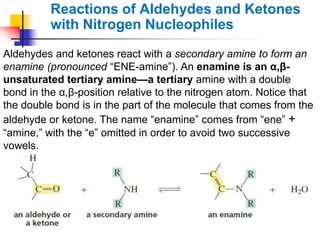
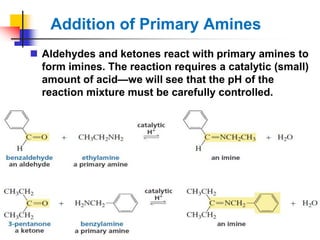
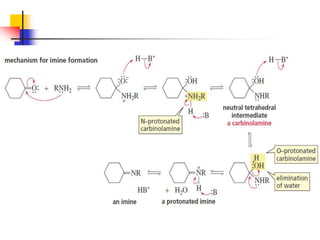
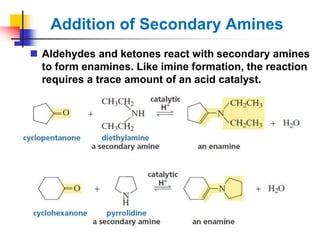
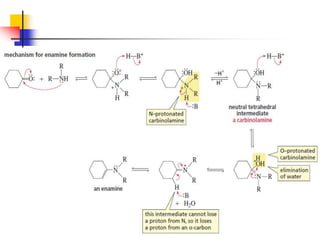
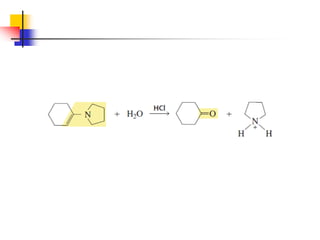
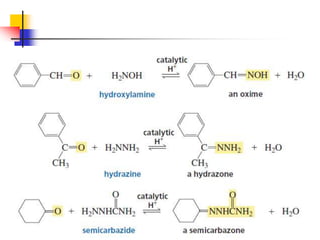
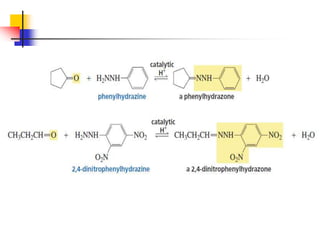
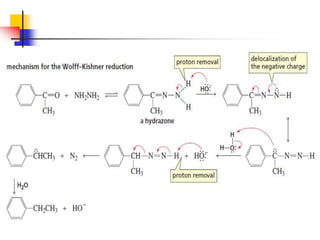
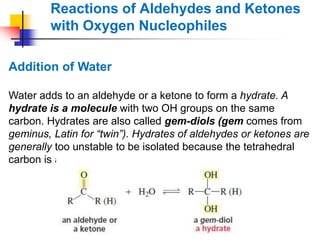
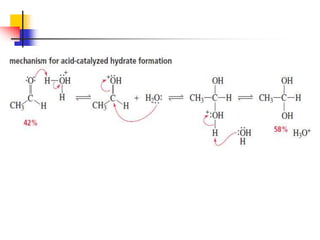
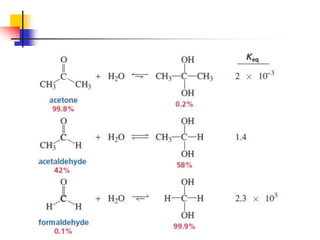
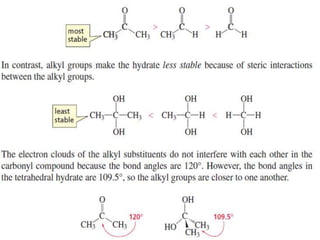
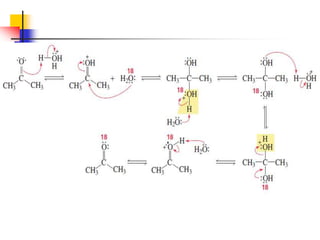
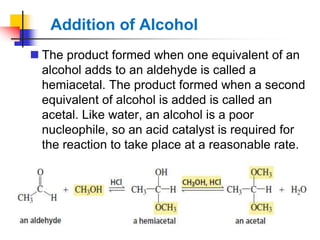
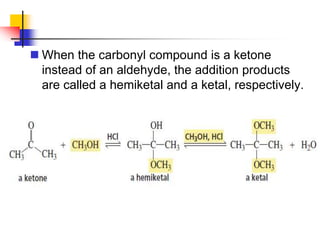
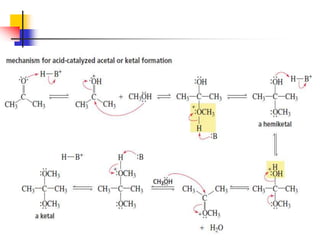
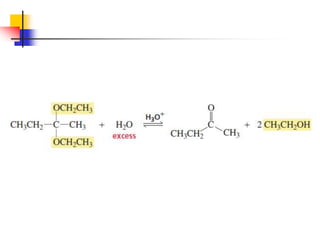
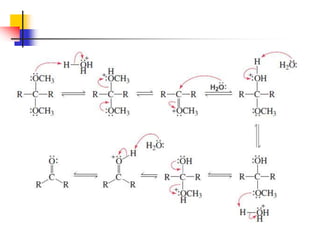
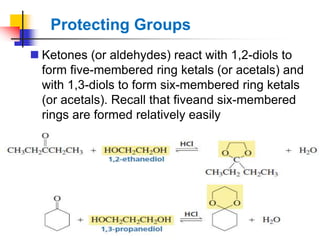
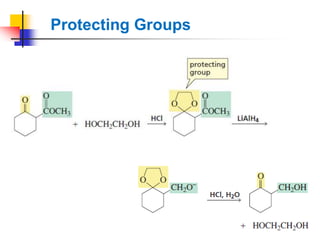
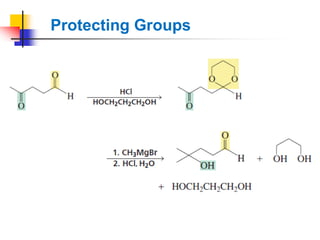
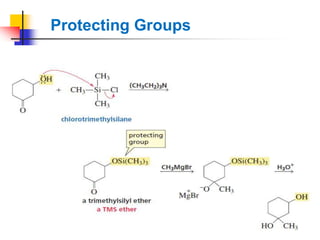
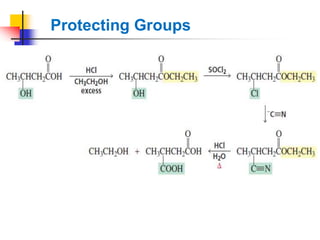
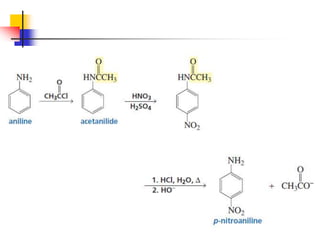
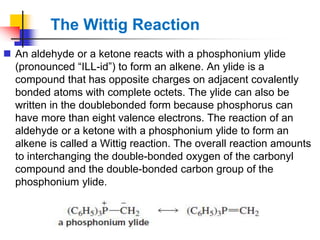
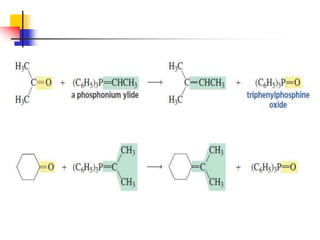
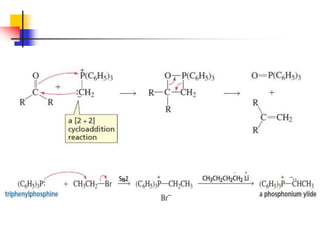
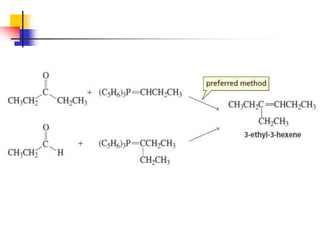
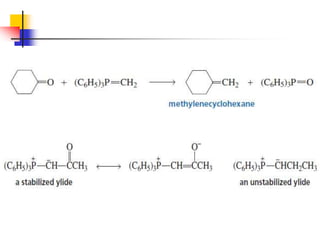
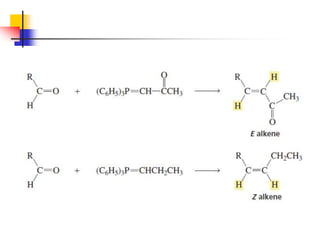
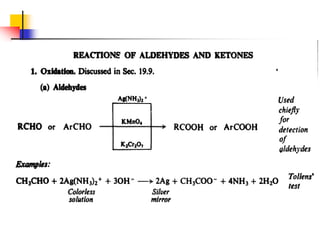
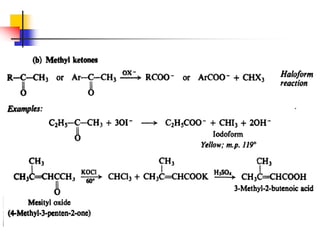
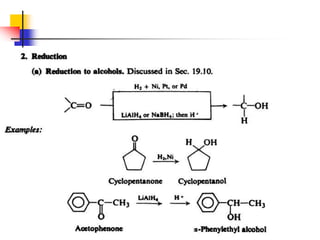
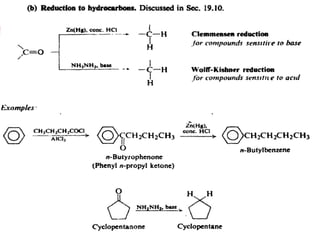
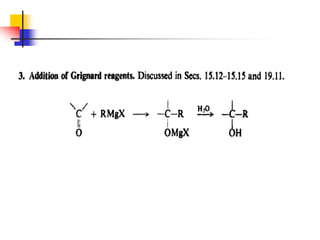
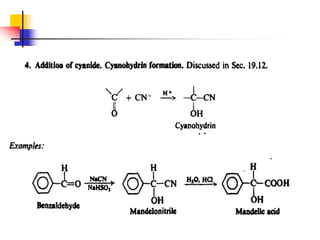
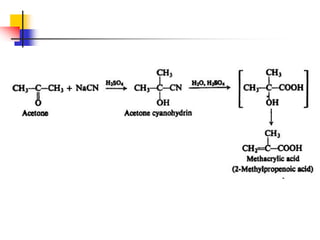
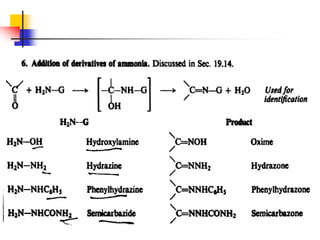
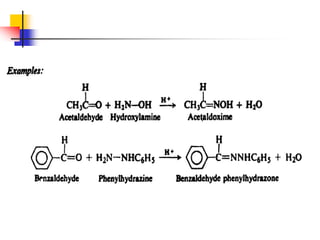
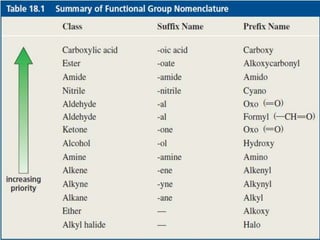
1) The document discusses various reactions of carbonyl compounds including aldehydes and ketones.
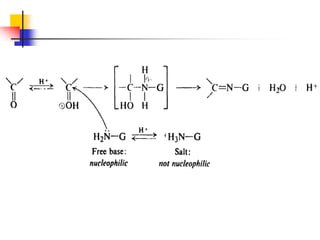
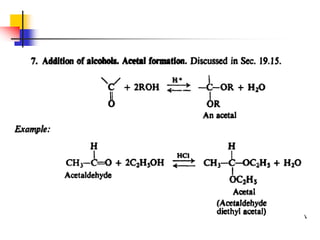
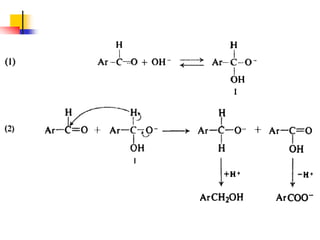
2) It describes reactions with nucleophiles like hydride ions, amines, alcohols and Grignard reagents that add to the carbonyl group.
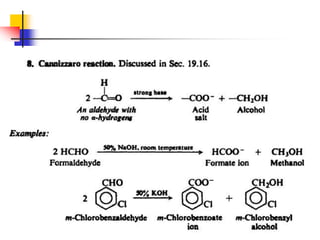
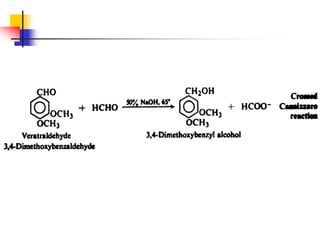
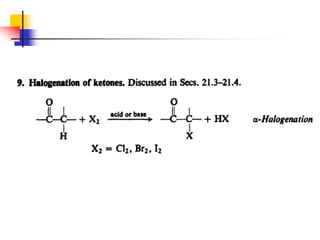
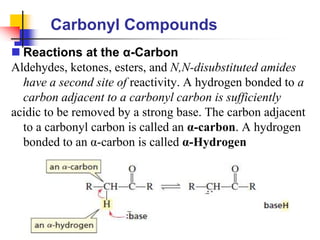
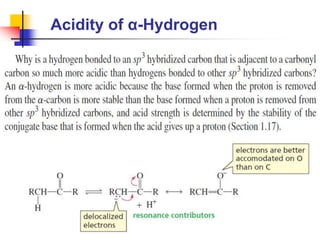
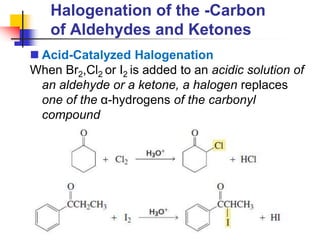
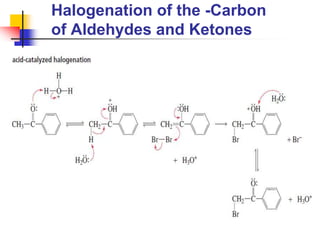
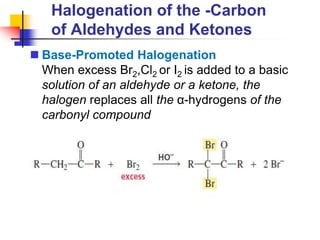
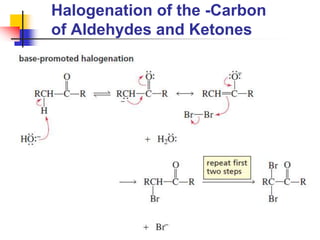
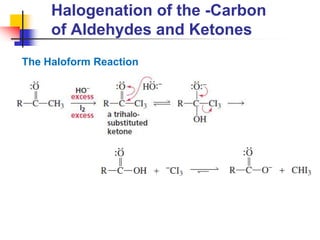
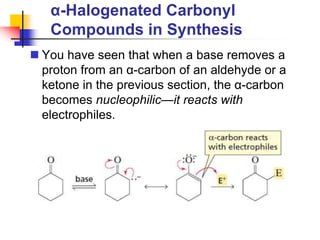
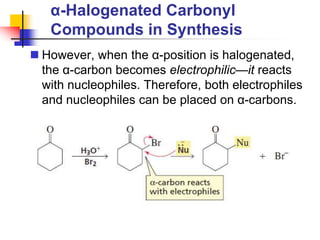
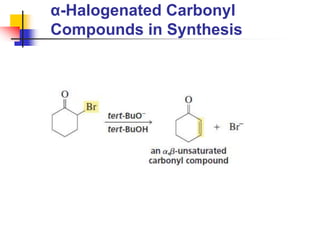
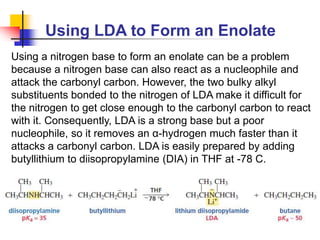
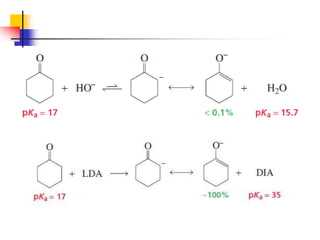
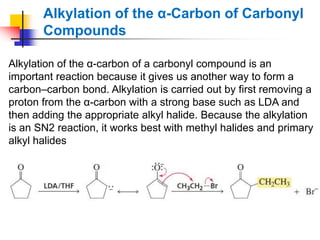
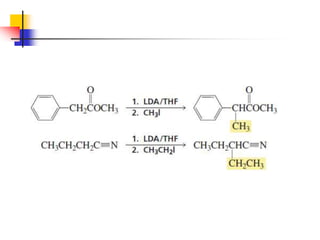
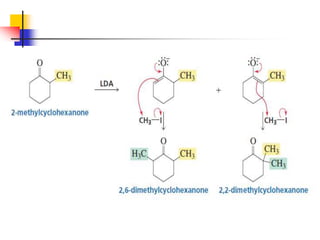
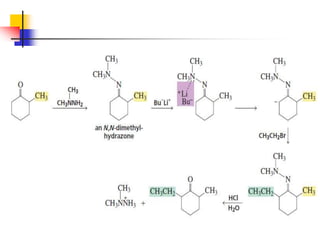
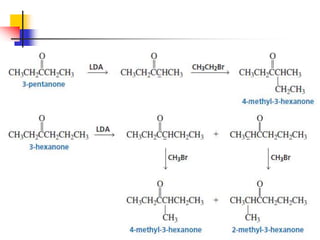
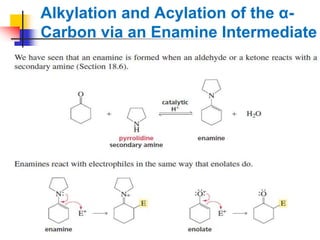
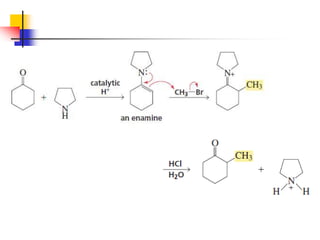
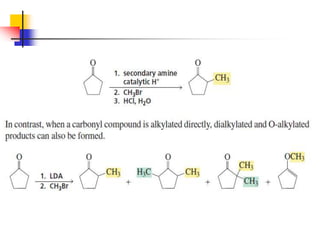
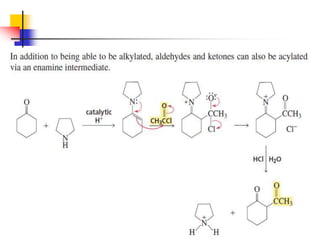
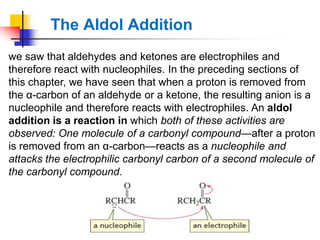
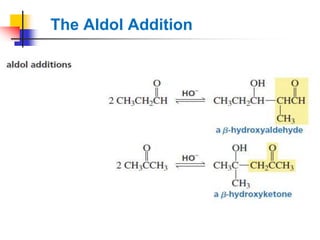
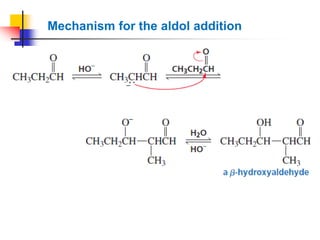
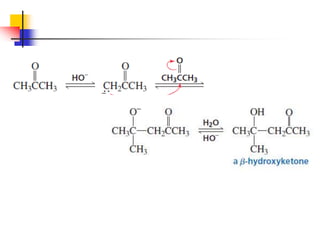
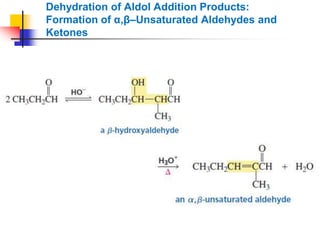
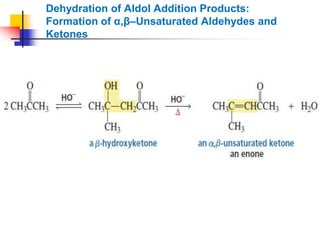
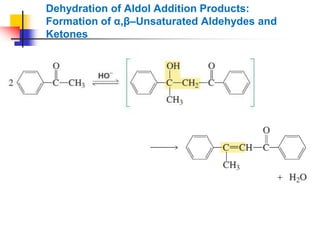
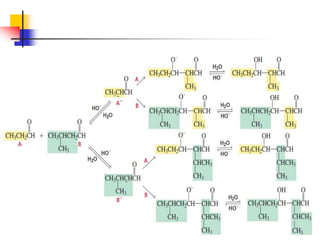
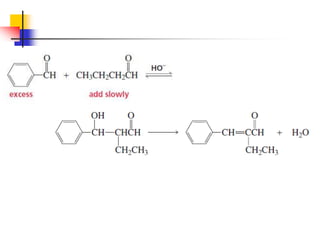
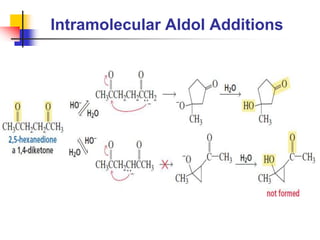
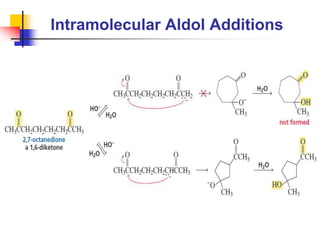
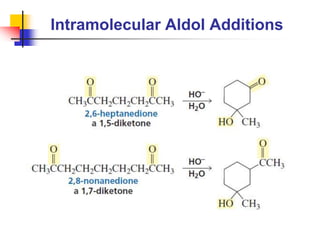
3) It also discusses reactions where the alpha-hydrogen of carbonyl compounds is removed, making the alpha-carbon nucleophilic and able to react with electrophiles via substitutions and additions. Important reactions covered include halogenation, alkylation and aldol additions.