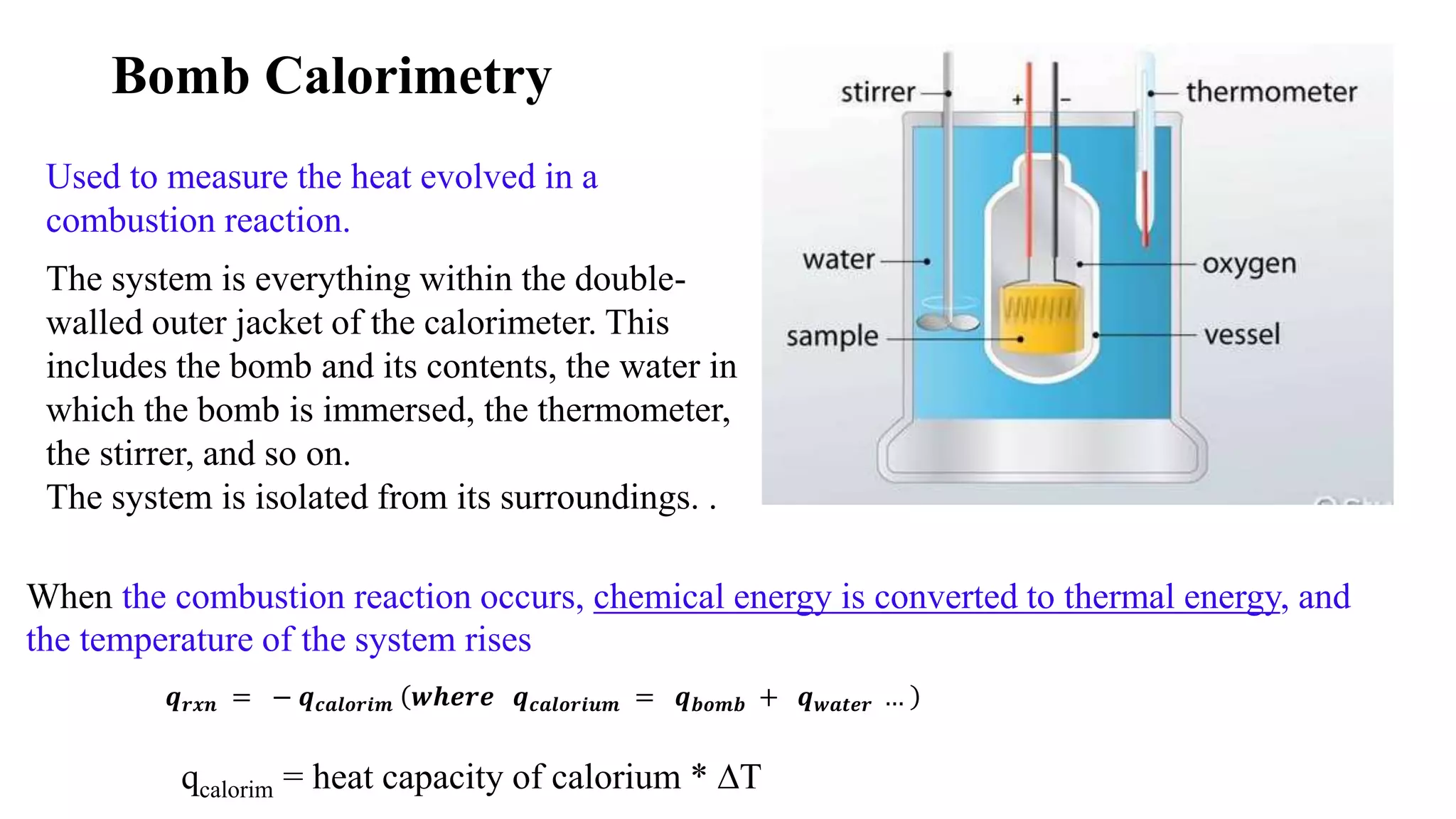
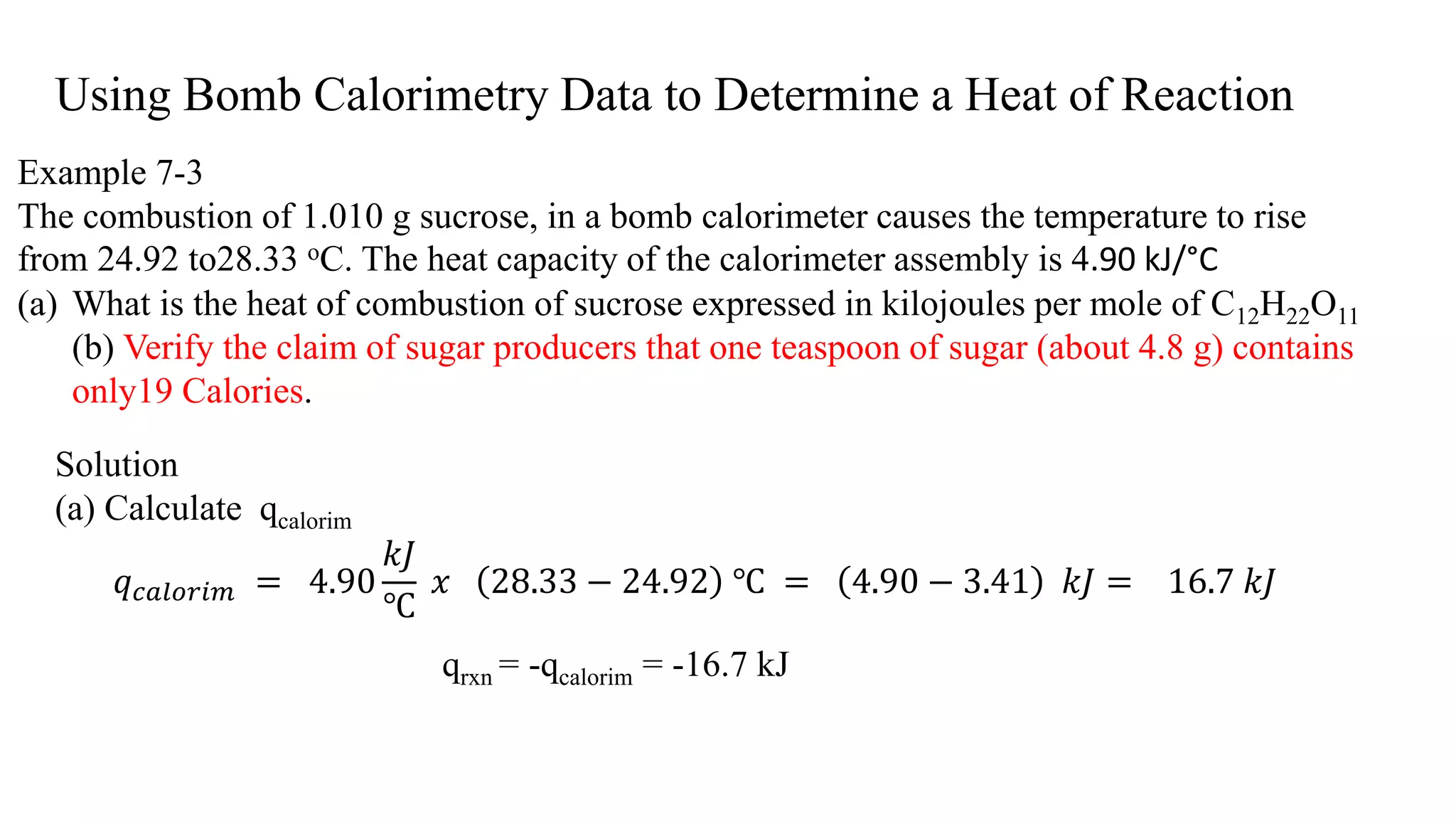
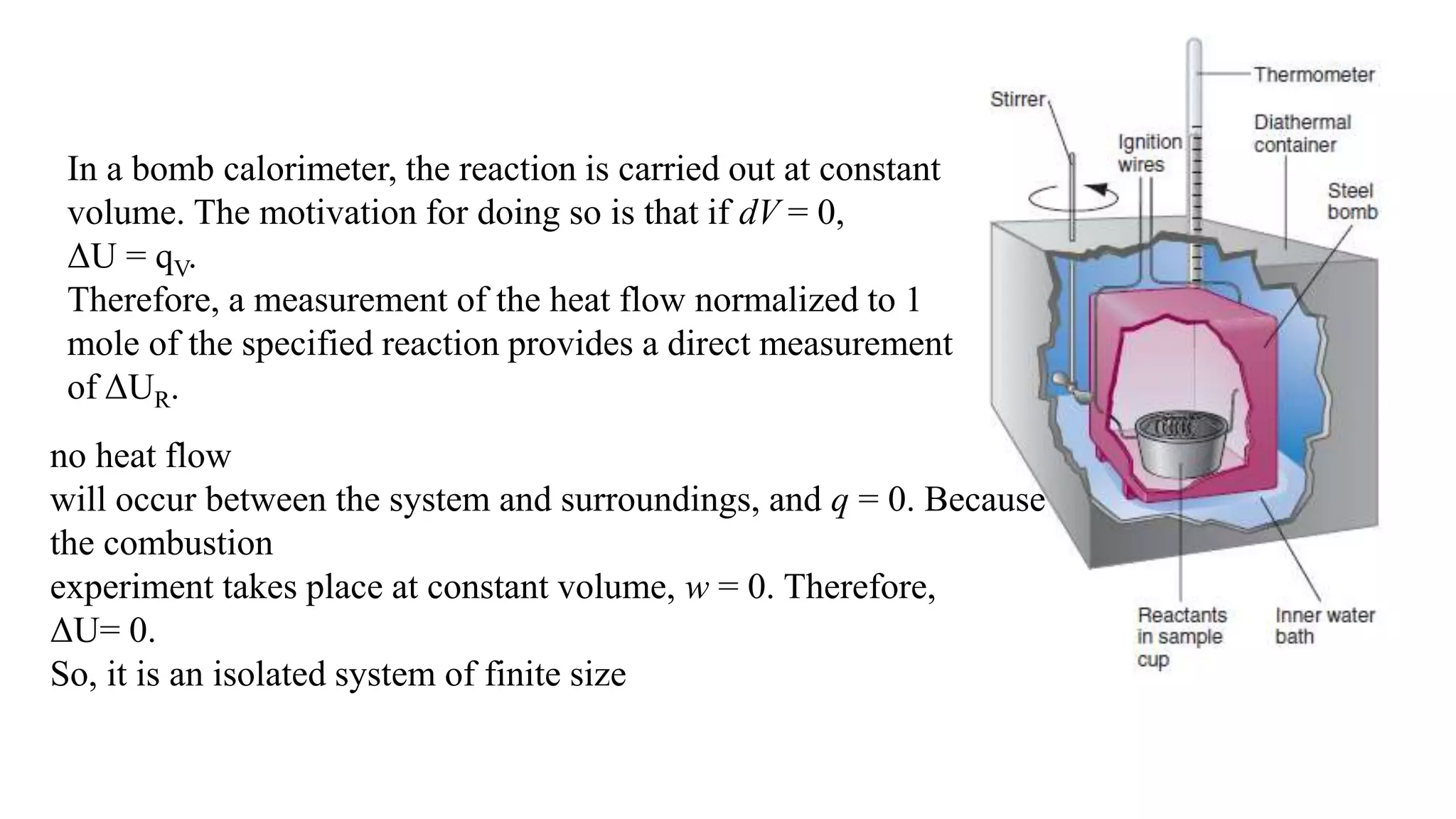
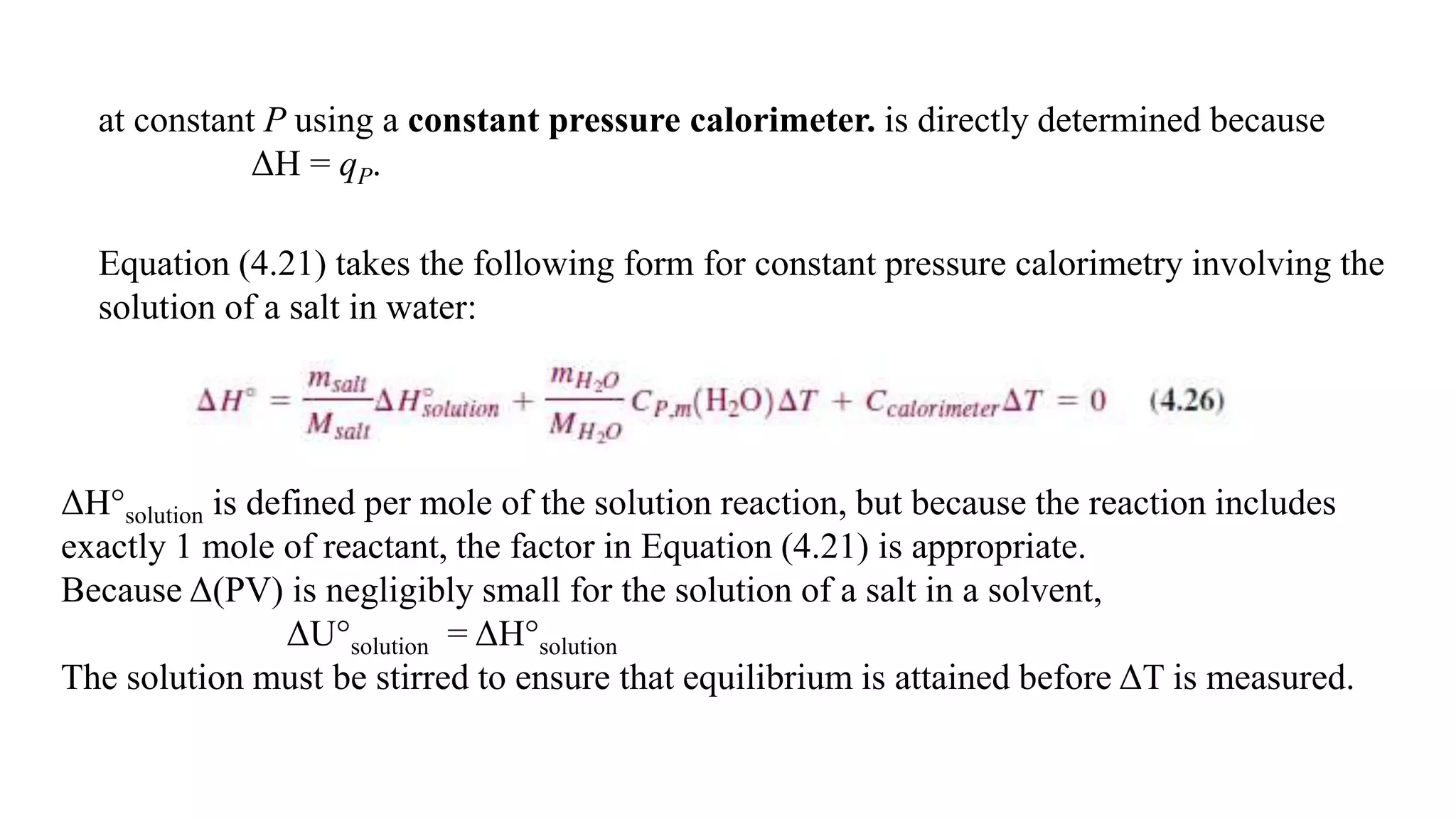
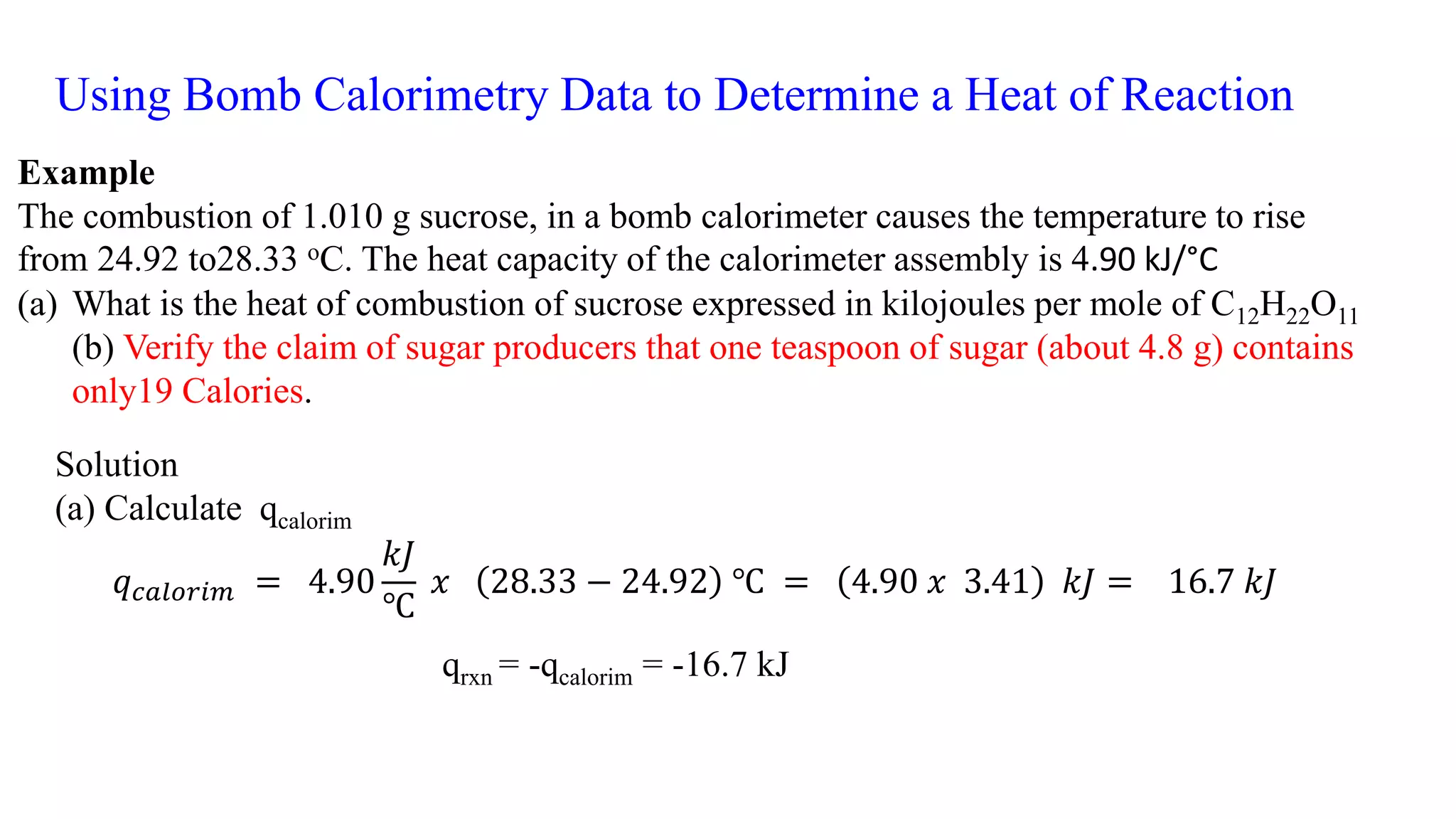
The document discusses bomb calorimetry, a method used to measure heat changes during chemical reactions, particularly combustion. It explains concepts such as heat of reaction, the difference between endothermic and exothermic reactions, and provides examples of calculating heat of combustion for substances like sucrose and octane. It also details the principles of work in relation to gas reactions and emphasizes the importance of measuring heat flow in isolated systems.