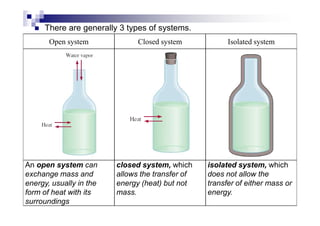
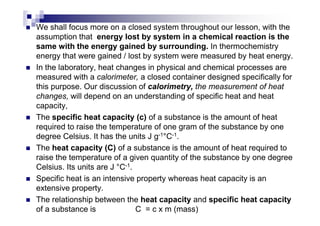
This document provides an introduction to thermochemistry and the key concepts of enthalpy, enthalpy change, and standard enthalpy of formation. It defines system and surroundings, and the three types of systems - open, closed, and isolated. The key points are:
- Enthalpy change (ΔH) is the difference in enthalpies between products and reactants and indicates whether a reaction is endothermic or exothermic.
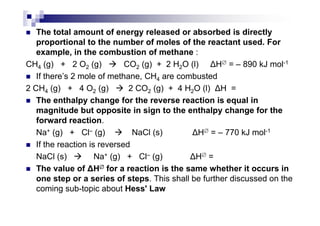
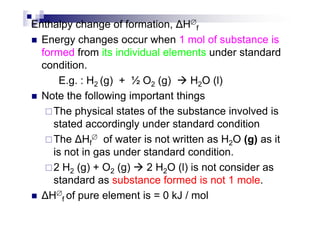
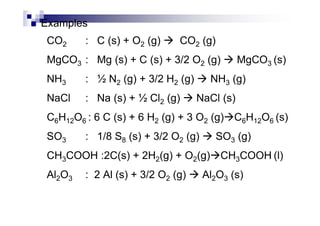
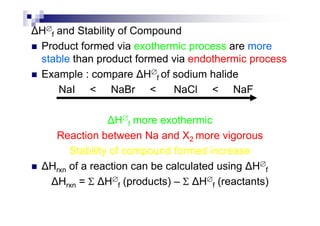
- Standard enthalpy of formation (H°f) is the enthalpy change when 1 mole of a substance is formed from its elements under standard conditions.
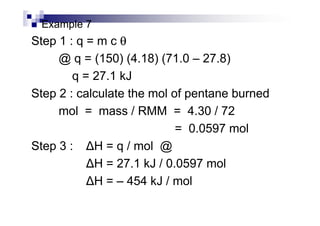
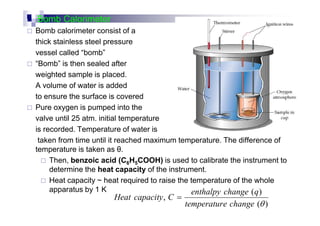
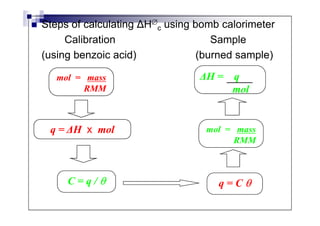
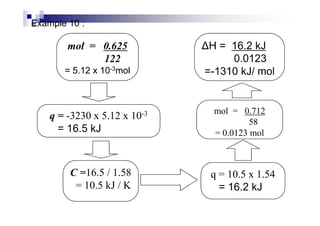
- Enthalpy of combustion (H°c) is the enthalpy change when 1 mole


![1.2 Enthalpy and Enthalpy Change
Measurement of energy transferred during chemical reaction is made
under control conditions. However, in a closed system, we assume that
there’s no changes in the volume of a system, hence no work is done
toward the heat change occur within the system. By that, we shall
deduce the energy transferred in a system is corresponding to the heat
transfer towards the surrounding. Heat transfer in this case is described
as enthalpy, H.
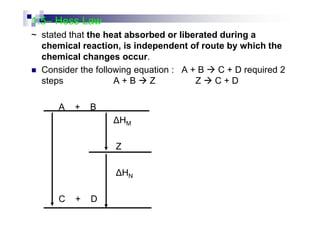
In a chemical reaction, where reactants products
The difference of energy changes occur on a chemical reaction is known
as enthalpy change, ∆H, as the difference between the enthalpies of the
products and the enthalpies of the reactants
H = [ΣΣΣΣ Hproduct – ΣΣΣΣ Hreactant].
Such enthalpy is also known as enthalpy change of reaction
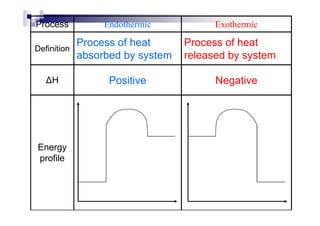
Since the enthalpy changes is a quantitative value use to measure the
difference by the heat given off before and after a reaction, so it may be
a positive value or negative value](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-5-320.jpg)
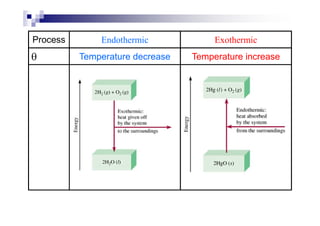
![Enthalpy – heat content of the system
Enthalpy changes ; H ~ heat changes occur
during a chemical reaction.
H = [ΣΣΣΣ Hproduct – ΣΣΣΣ Hreactant]
Unit = kJ mol-1.
Σ Hproduct > Σ Hreactant Σ Hproduct < Σ Hreactant
H = positive (+ve) H = negative (–ve)
Endothermic exothermic](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-6-320.jpg)
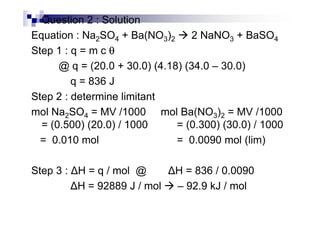
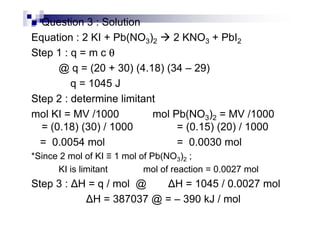
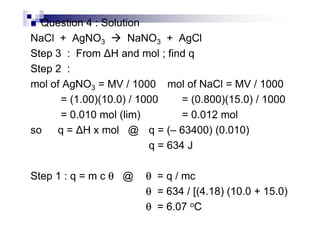
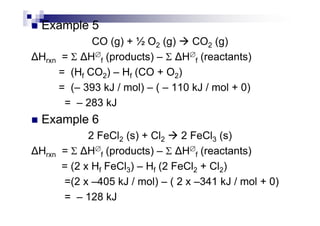
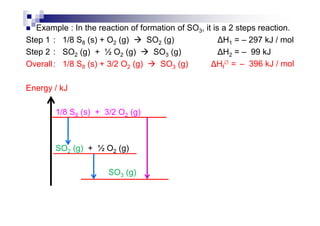
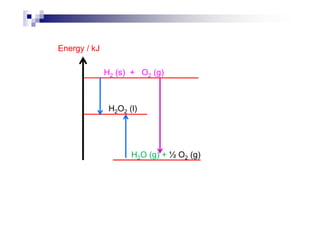
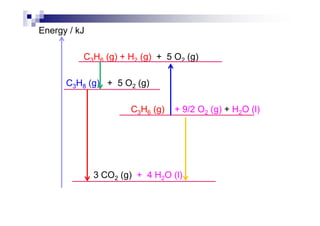
![Examples
C : C (s) + O2 (g) CO2 (g) [= H∅
fof CO2]
H2 : H2 (g) + ½ O2 (g) H2O (l) [= H∅
fof H2O]
C2H5COOH : C2H5COOH (l) + 7/2 O2 (g)
3 CO2 (g) + 3 H2O (l)
C2H5OH : C2H5OH (l) + 3 O2 (g) 2 CO2 (g) + 3 H2O (l)
Mg : Mg (s) + ½ O (g) MgO (s)Mg : Mg (s) + ½ O2 (g) MgO (s)
P : P4 (s) + 5 O2 (g) P4O10 (s)
Al : Al (s) + 3/4 O2 (g) ½ Al2O3 (s)
C6H12O6 : C6H12O6 (s) + 6 O2 (g) 6 CO2 (g) + 6 H2O (l)](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-22-320.jpg)


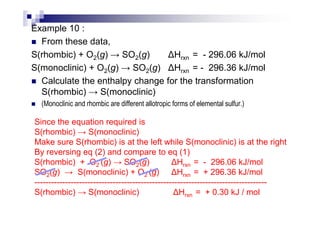
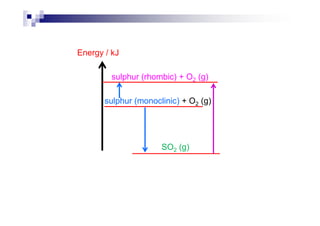
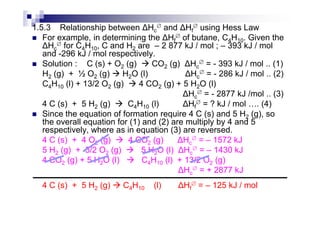

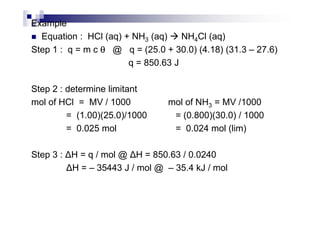
![H∅
neut for weak acid or weak alkali reaction.
If H∅
neut is ≠ 57.3 kJ / mol depend on :
the example above, it can be tell that, the H∅
neut for
weak acid and strong alkali is ≠ - 57.3 kJ / mol. This is
due to, some heat is absorbed by CH3COO-H to break the
O-H to form hydrogen ion. Therefore, it is less exothermic
than the expected value.
Basicity of an acid : HCl H+ + Cl– [monoproctic acid]
H2SO4 2 H+ + SO4
2- [diproctic acid]
H3PO4 3 H+ + PO4
3- [triproctic acid]H3PO4 3 H + PO4 [triproctic acid]
For example, when NaOH (aq) react with H2SO4 (aq)
Stage 1 :H2SO4 (aq) + NaOH (aq) NaHSO4 (aq) + H2O (l)
H∅
neut = –61.95 kJ / mol
Stage 2 : NaHSO4 (aq) + NaOH (aq) Na2SO4 (aq) + H2O (l)
H∅
neut = –70.90 kJ / mol
Overall : 2 NaOH (aq) + H2SO4 (aq) Na2SO4 (aq) + 2 H2O (l)
H∅
neut = [-61.95 + (-70.90)]= - 132.85 kJ](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-45-320.jpg)





![∆Hatom of Na
Na (g) + ½ Cl2 (g)
∆HIE of Na
Na+ (g) + ½ Cl2 (g) + e-
∆Hatom of Cl
Na+ (g) + Cl (g) + e-
∆HEA of Cl
Na+ (g) + Cl- (g)
∆HLEof NaCl
Na (s) + ½ Cl2 (g)
NaCl (s)
∆Hf of NaCl
∆Hatom of Na ∆HLEof NaCl
H∅
f = H∅
LE + [ H∅
atom Na + H∅
atom Cl + H∅
1st IE Na + H∅
1st EA Cl]
H∅
LE = (-411) – [(+108) + (+121) + (+494) + (-364)]
= – 770 kJ/mol](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-57-320.jpg)
![∆H of Ca
Ca (g) + Cl2 (g)
∆H1st IE of Ca +
∆H2nd IE of Ca
Ca2+ (g) + Cl2 (g) + 2 e-
2 x ∆Hatom of Cl
Ca2+ (g) + 2 Cl (g) + 2 e-
2 x ∆HEA of Cl
Ca2+ (g) + 2 Cl- (g)
∆H of CaCl
Ca (s) + Cl2 (g)
CaCl2 (s)
∆Hf of CaCl2
∆Hatom of Ca ∆HLEof CaCl2
H∅
f = H∅
LE + [ H∅
atom Ca + 2 H∅
atom Cl + H∅
1st IE Ca +
H∅
2nd IE Ca + 2 H∅
1st EA Cl]
H∅
LE = (-795) – [(+132) + 2(+121) +(+590) +(1150) + 2(-364)]
= – 2181 kJ/mol](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-58-320.jpg)
![2 X ∆H of K
2 K (g) + ½ O2 (g)
2 X ∆H1st IE of K
2 K+ (g) + ½ O2 (g) + 2 e-
∆Hatom of O
2 K+ (g) + O (g) + 2 e-
∆HLEof K2O
∆H1st EA +
∆H2nd EA
2 K+ (g) + O2- (g)
2 K (s) + ½ O2 (g)
K2O (s)
∆Hf of K2O
2 X ∆Hatom of K
H∅
f = H∅
LE + [2 H∅
atom K + ½ H∅
BE O + 2 H∅
1st IE K +
H∅
1st EA O + H∅
2nd EA O]
H∅
LE = (-362) – [2(+129) + ½(+498) + 2(418) + (-141)+(+844)]
= – 2408 kJ/mol](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-59-320.jpg)
![Mg (g) + ½ O2 (g)
∆H1st IE of Mg +
∆H2nd IE of Mg
Mg2+ (g) + ½ O2 (g) + 2 e-
∆Hatom of O
Mg2+ (g) + O (g) + 2 e-
∆HLEof MgO
∆H1st EA +
∆H2nd EA of O
Mg2+ (g) + O2- (g)
Mg (s) + ½ O2 (g)
MgO (s)
∆Hf of MgO
∆Hatom of Mg
H∅
f = H∅
LE + [ H∅
atom Mg + ½ H∅
BE O + H∅
1st IE Mg +
H∅
2nd IEMg + H∅
1st EA O + H∅
2nd EA O]
H∅
LE = (-612) – [(+146) + ½(+498)+(736) + (1450) + (-141)+(+844)]
= – 3896 kJ/mol](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-60-320.jpg)
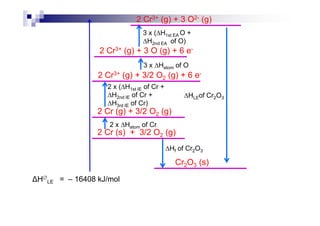



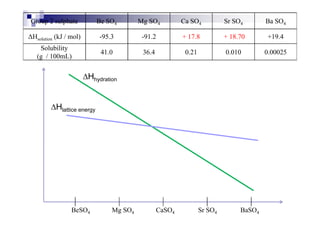
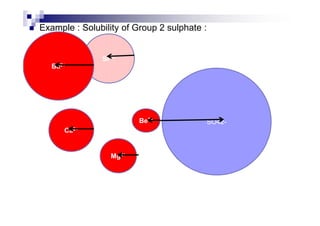
![1.12 Enthalpy change of solution, Hsoln
Energy change when 1 mole of solute is dissolved in a large
excess water to form an infinite dilute solution.
For ionic substance : MX (s) + water M+ (aq) + X- (aq)
Some covalent subs : C6H12O6 (s) + water C6H12O6 (aq)
Hsoln is determined by Hhyd and HLE
HLE : M+ (g) + X- (g) MX (s) [reverse]
H : M+ (g) + X- (g) + water M+ (aq) + X- (aq)Hhyd : M+ (g) + X- (g) + water M+ (aq) + X- (aq)
– HLE : MX (s) M+ (g) + X- (g)
MX (s) + water M+ (aq) + X- (aq)
As a conclusion, Hsoln = Hhyd + (– HLE)
If Hsoln = - ve, then the salt is soluble in water
If Hsoln = + ve, then the salt is insoluble in water](https://image.slidesharecdn.com/chemistryform6sem201a-150220030450-conversion-gate01/85/Inorganic-Chemistry-Thermochemistry-66-320.jpg)