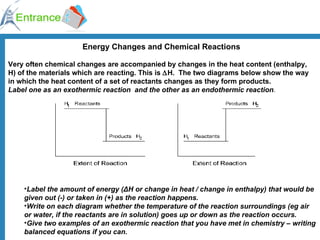
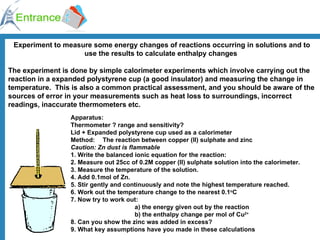
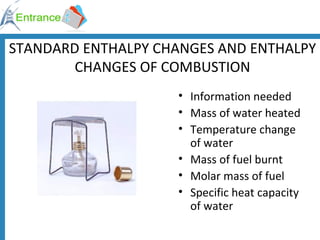
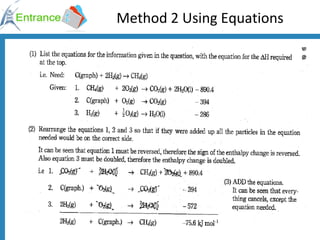
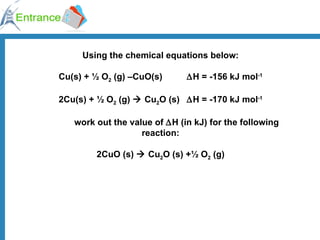
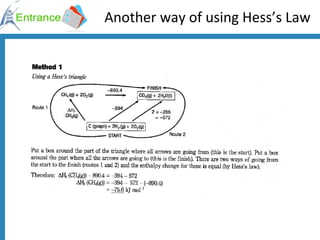
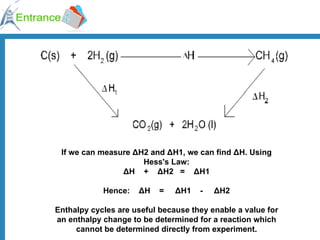
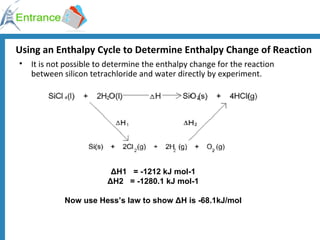
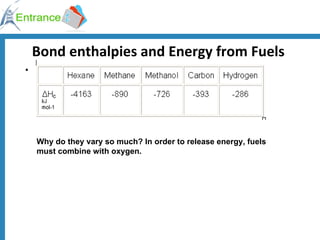
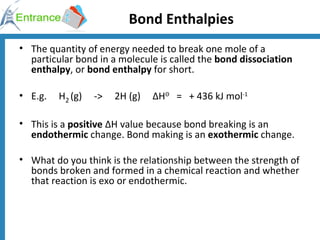
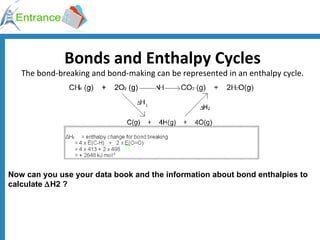
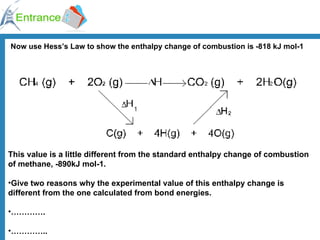
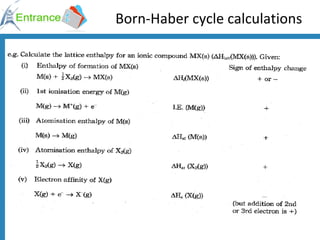
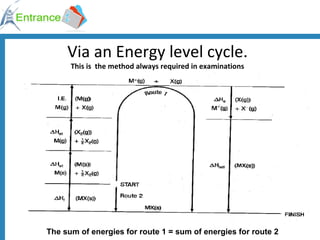
The document discusses energy changes that occur during chemical reactions. It defines exothermic and endothermic reactions, and standard enthalpy change. It provides examples of how to calculate enthalpy changes from experimental temperature change data using concepts like specific heat capacity. Hess's law, which states the enthalpy change of a reaction is equal to the sum of enthalpy changes of the steps in a reaction mechanism, is also introduced.