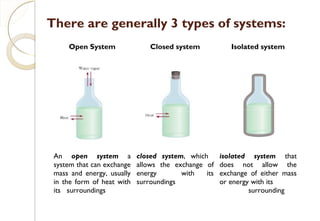
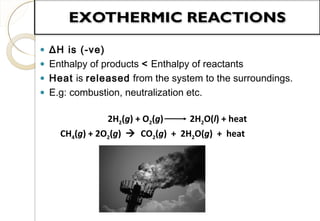
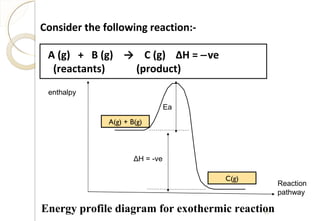
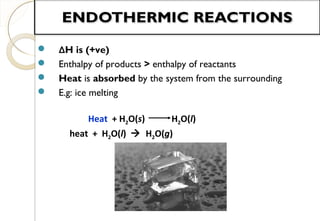
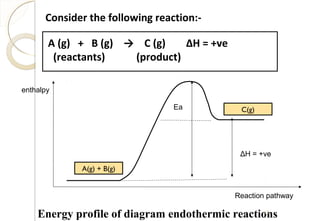
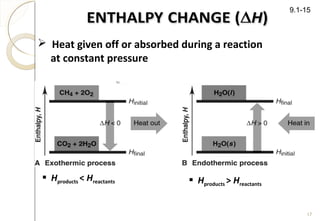
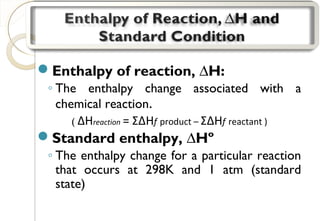
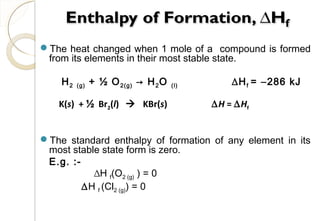
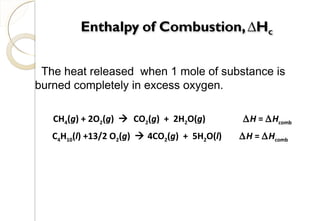
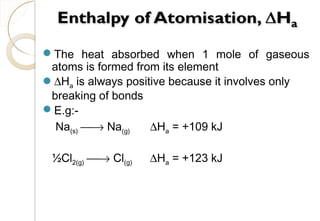
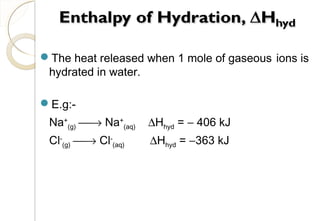
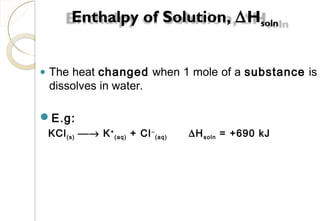
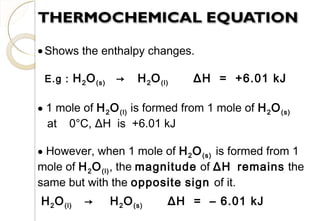
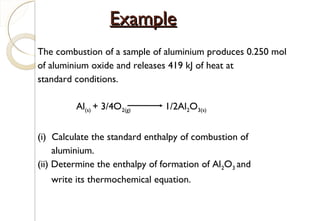
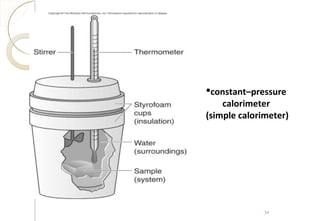
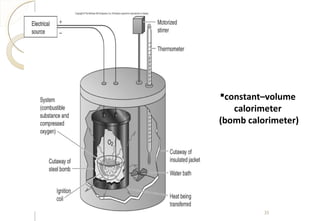
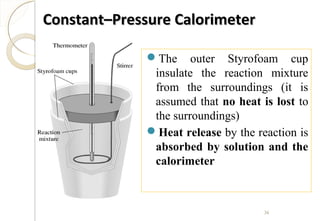
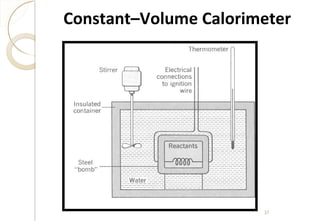
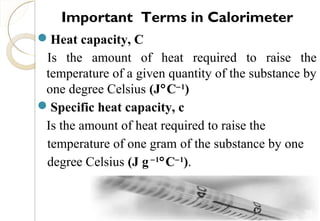
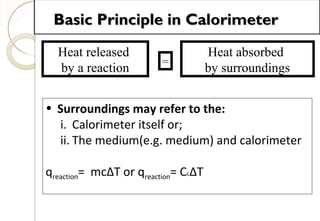
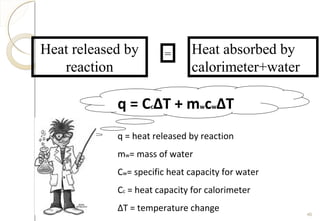
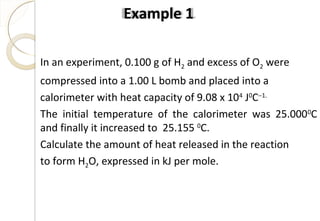
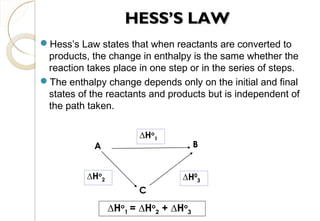
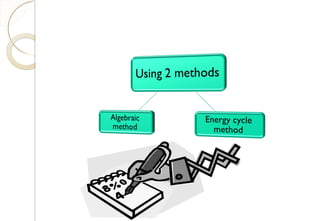
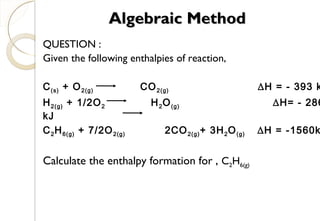
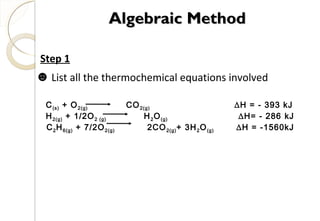
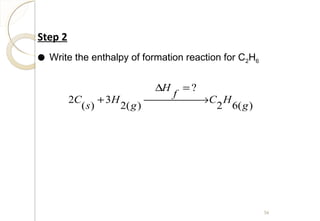
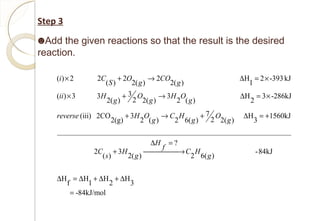
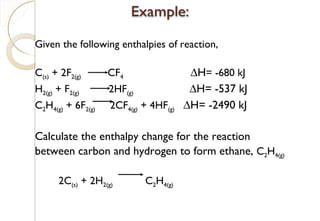
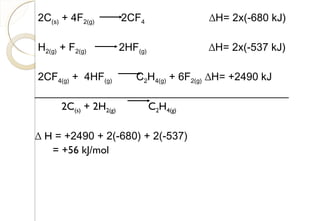
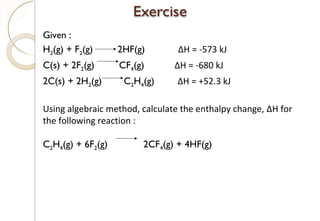
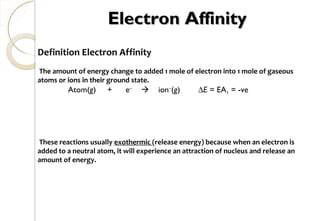
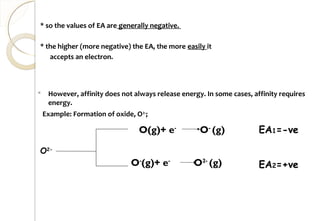
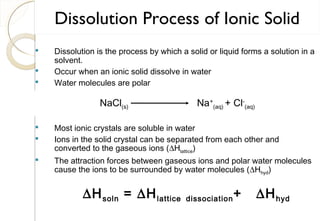
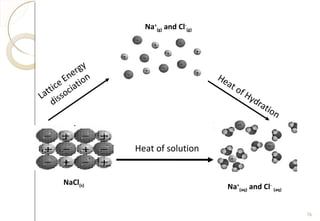
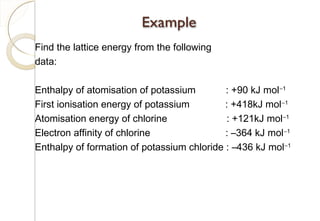
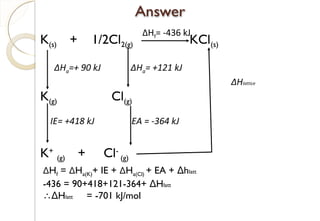
This document discusses thermo chemistry and key concepts like enthalpy, calorimetry, and Hess's law. It begins by defining open, closed, and isolated systems and explains endothermic and exothermic reactions using energy profile diagrams. It then discusses enthalpy and defines standard enthalpy. Various types of enthalpies are defined like enthalpy of formation, combustion, atomization, and more. Calorimetry is explained as a method to measure heat changes in reactions using calorimeters. Hess's law is introduced as the principle that the enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.








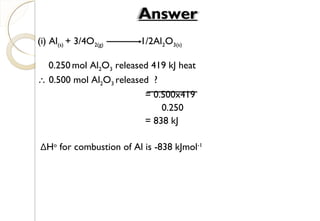
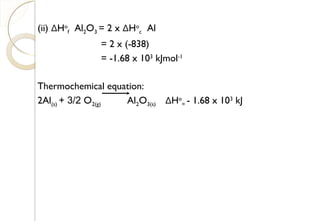

















![Calculation:Calculation:
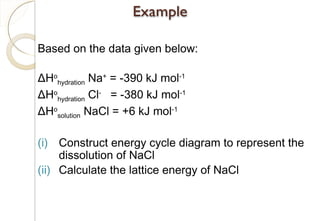
[ ]
( )[ ]
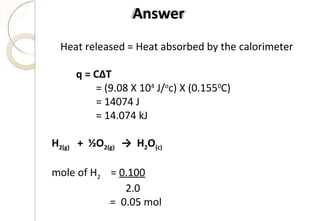
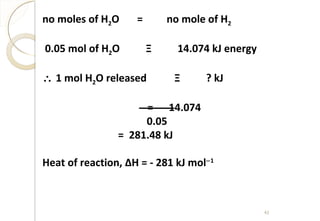
kJ777H
kJ364kJ122kJ500kJ108kJ411H
EAHIEHHH
HEAHIEHH
lattice
lattice
)Cl(aS
0
flattice
lattice)Cl(aS
0
f
−=∆
−++++−−=∆
+∆++∆−∆=∆
∆++∆++∆=∆
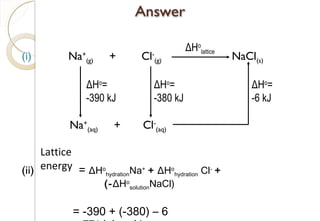
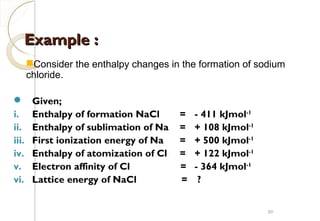
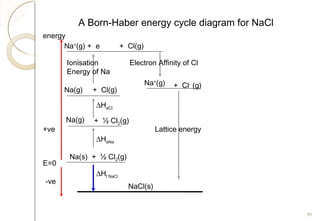
From Hess’s Law:
∆Hf NaCl = ∆HaNa + ∆HaCl +IENa +
EACl + Lattice Energy
91](https://image.slidesharecdn.com/8-151029034122-lva1-app6891/85/8-0-thermochemistry-student-s-copy-91-320.jpg)
