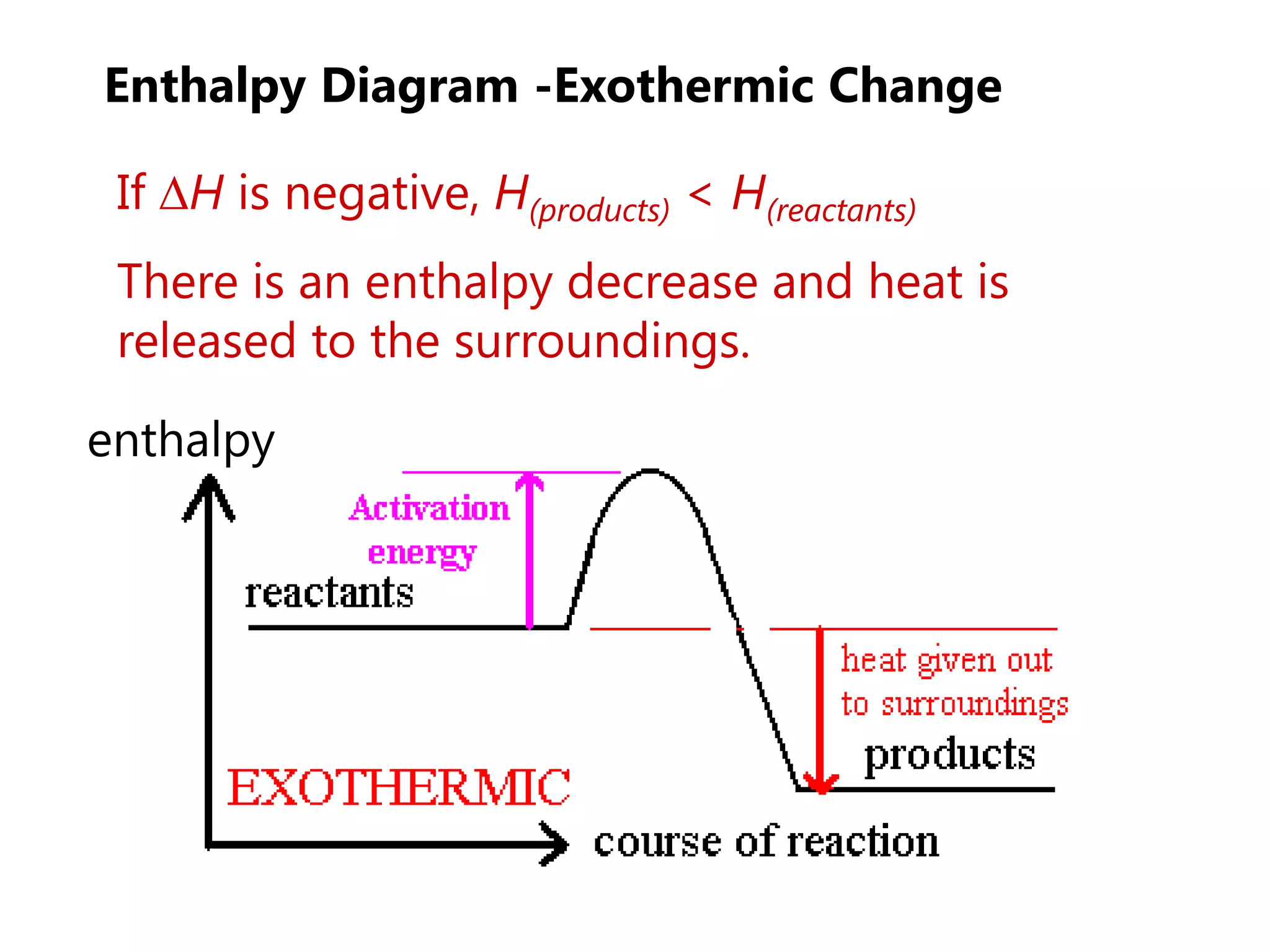
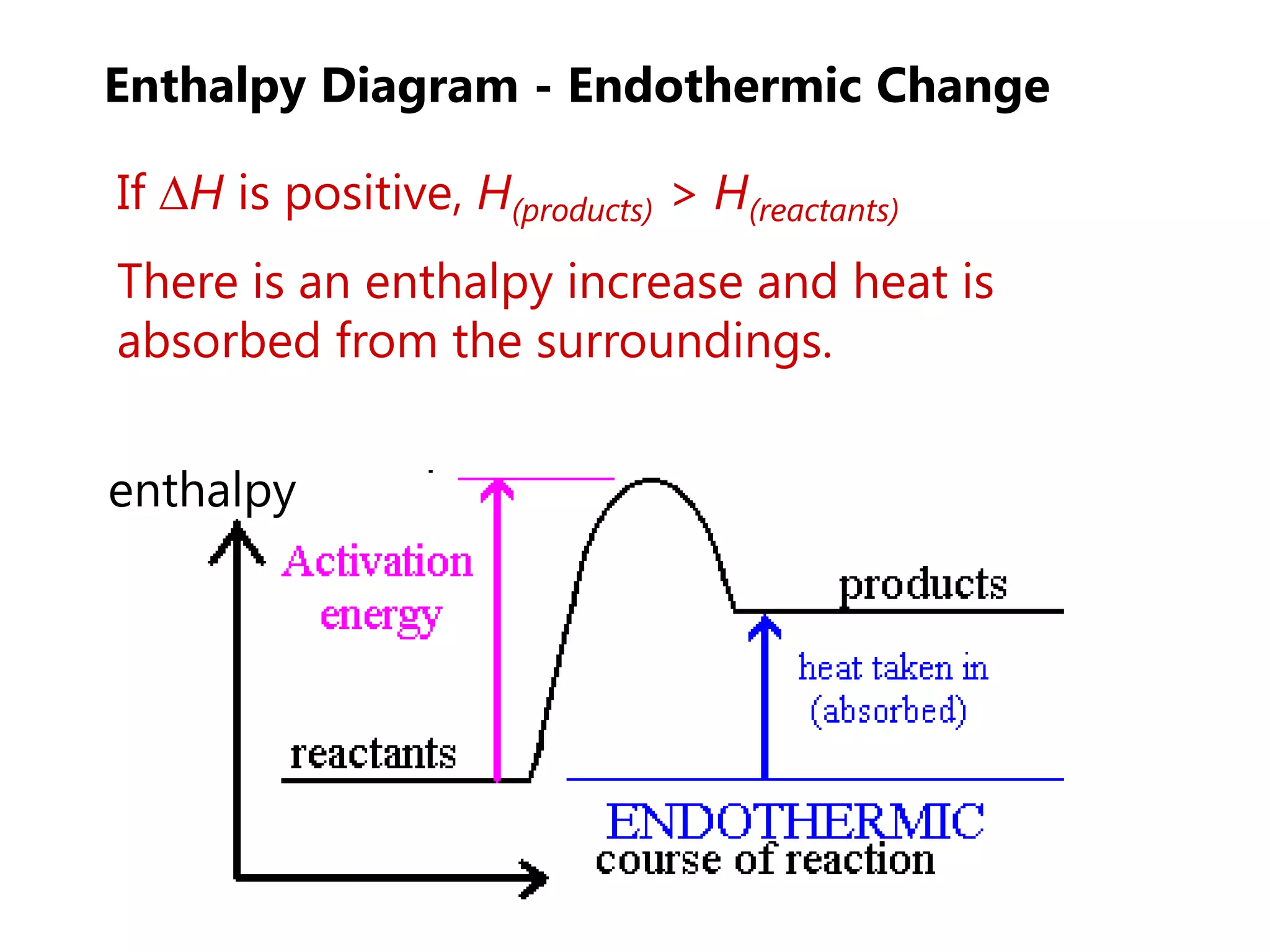
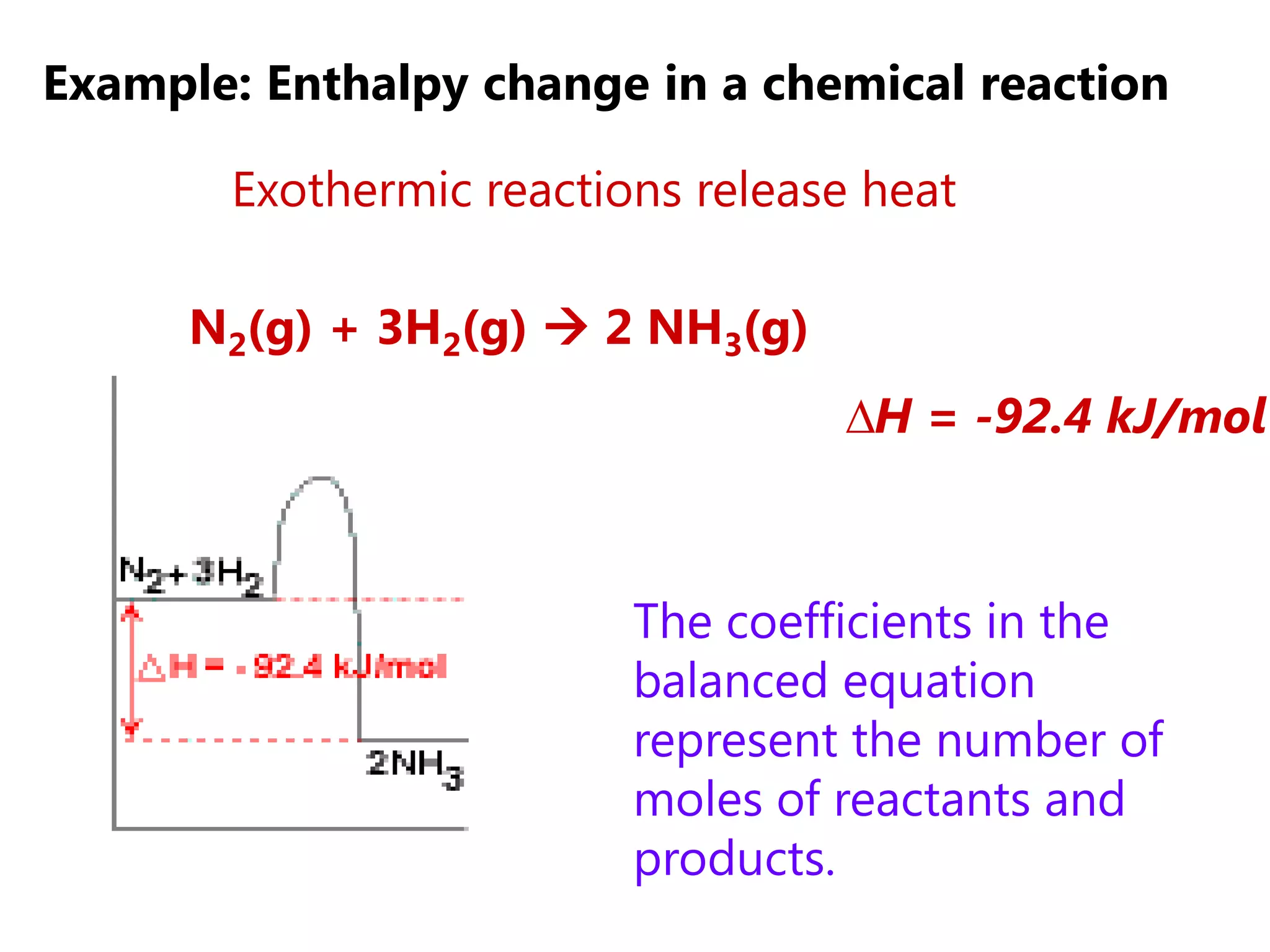
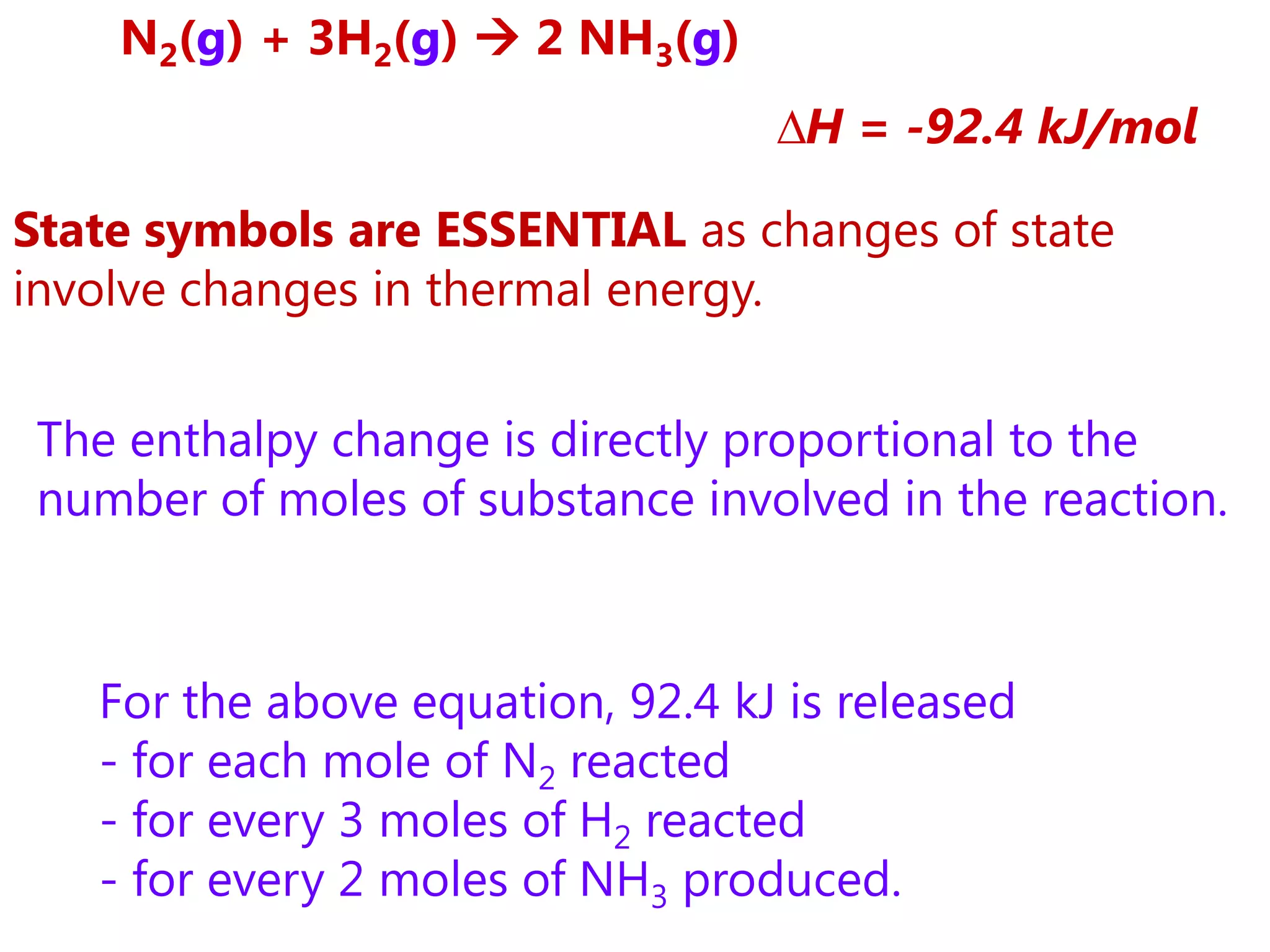
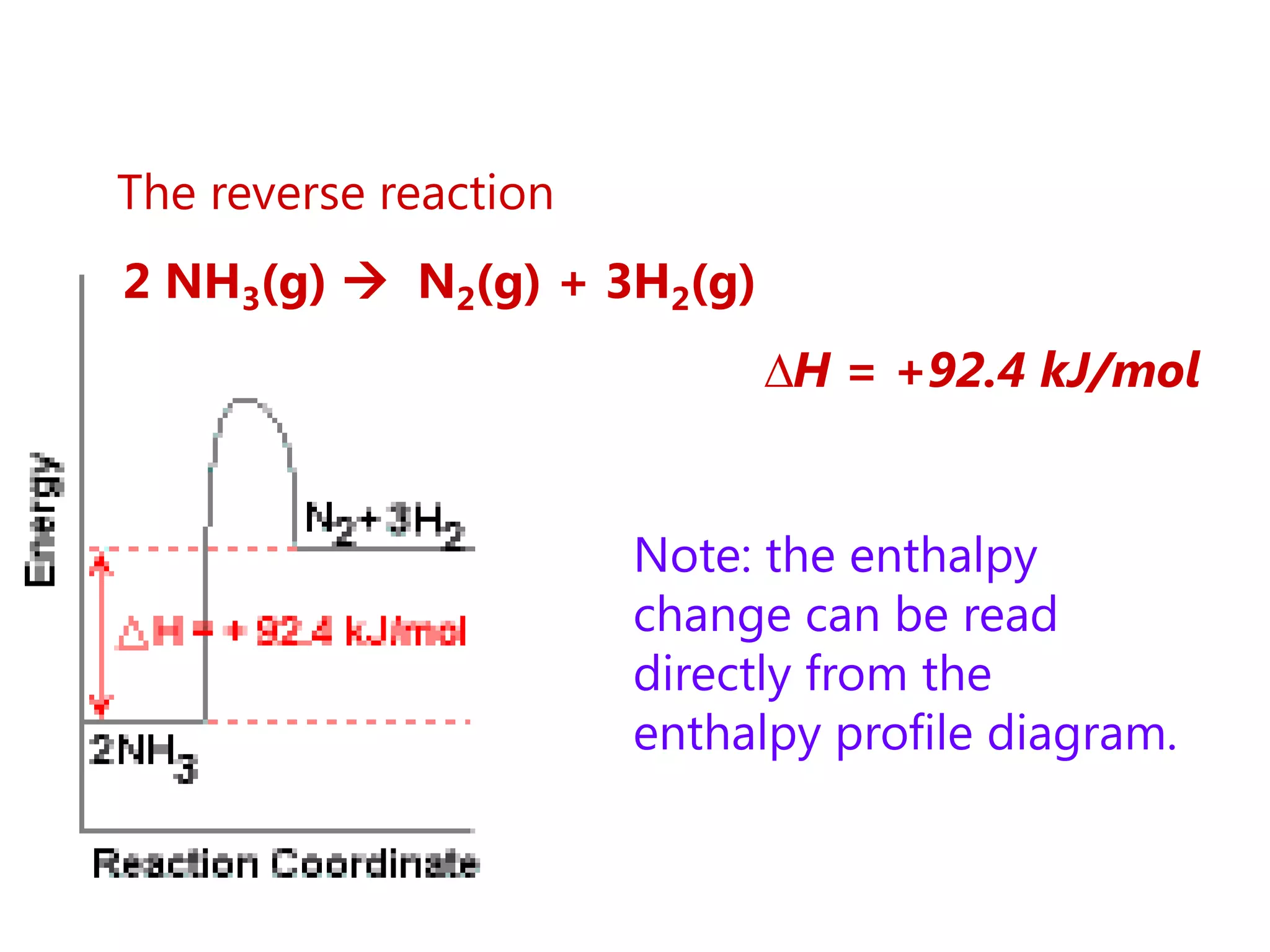
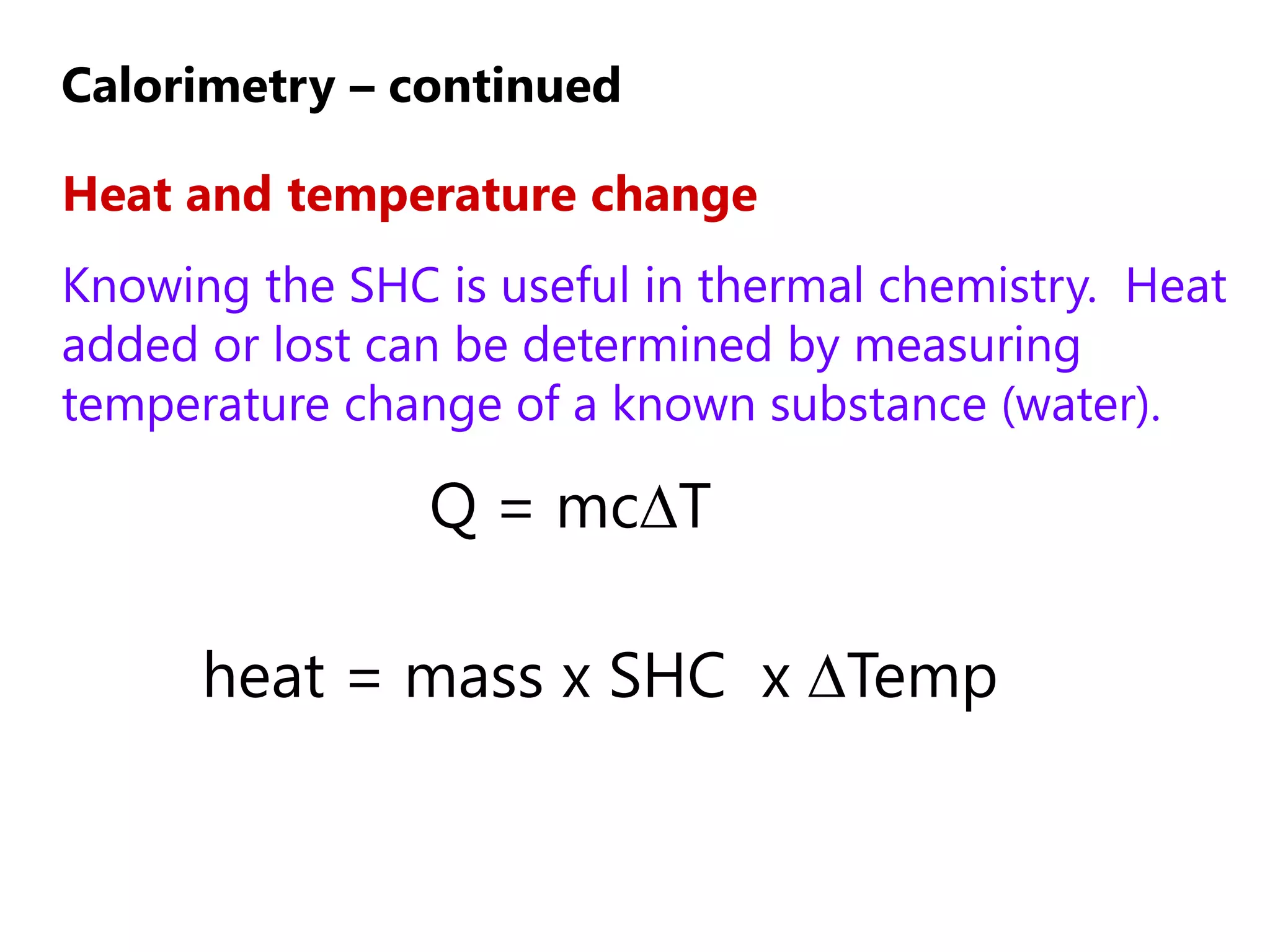This document provides an overview of exothermic and endothermic reactions, calorimetry, and how to calculate enthalpy changes. It discusses key concepts like:
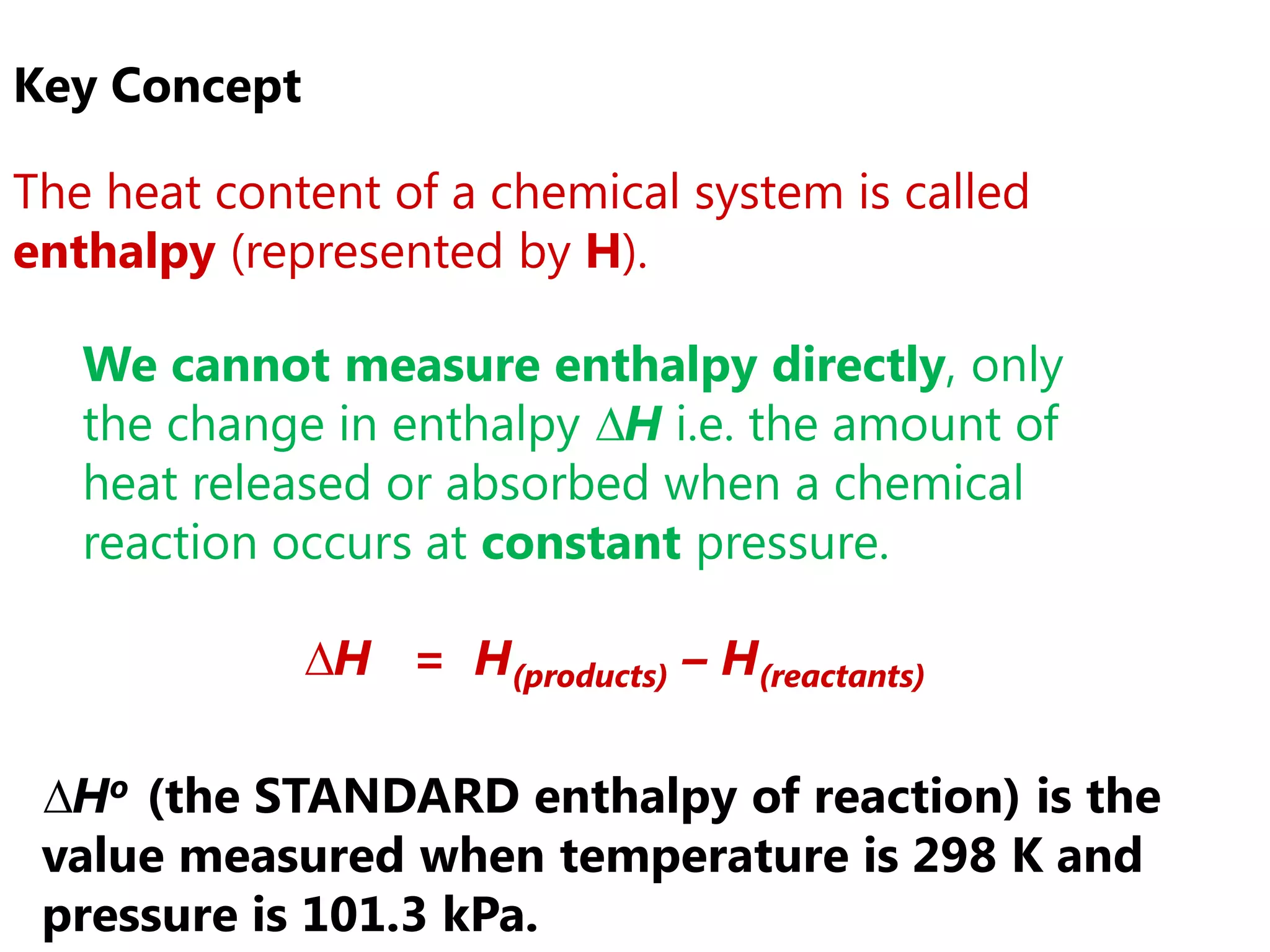
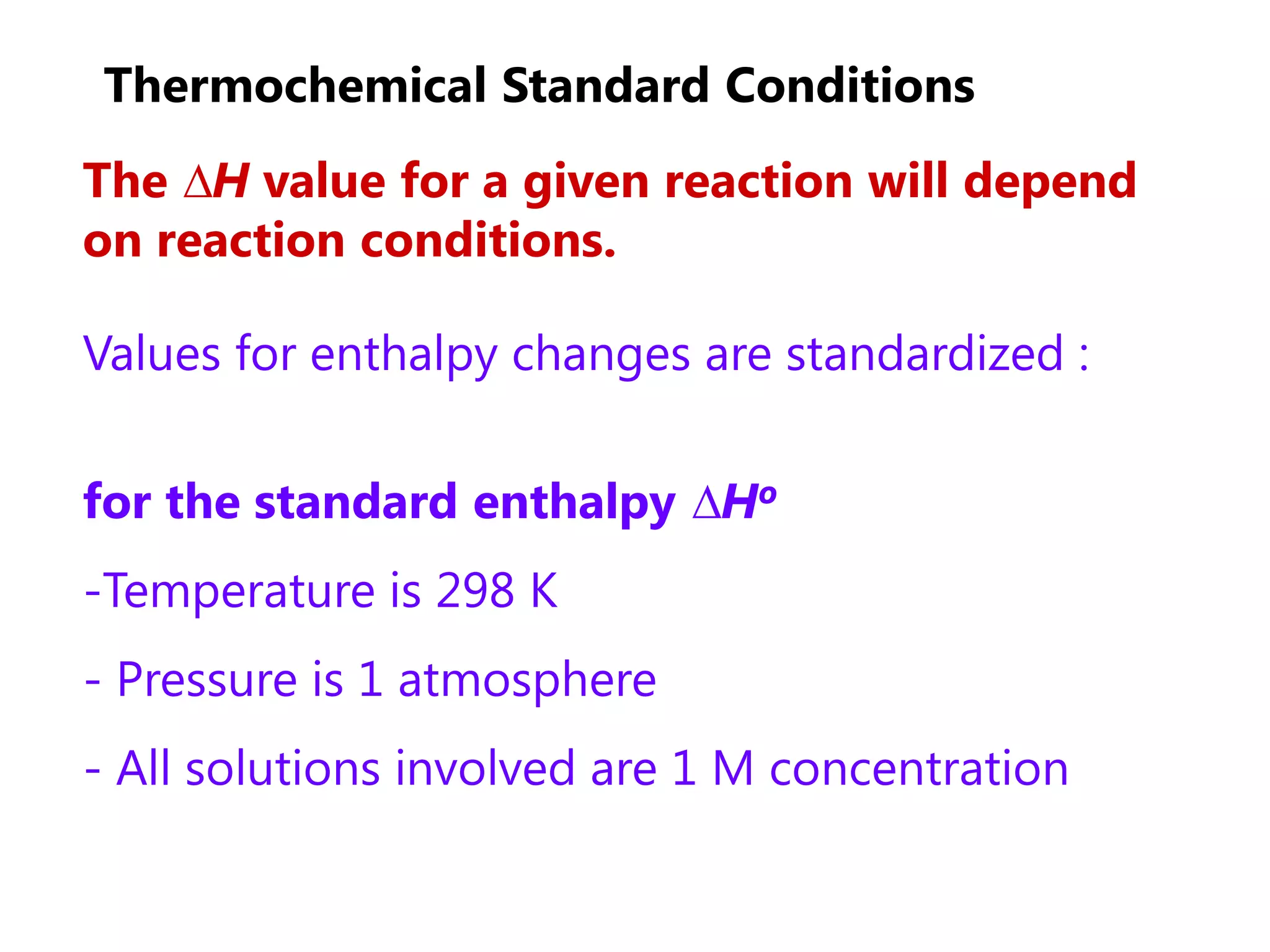
1) The standard enthalpy change (ΔH°) is the enthalpy change measured under standard conditions of temperature and pressure.
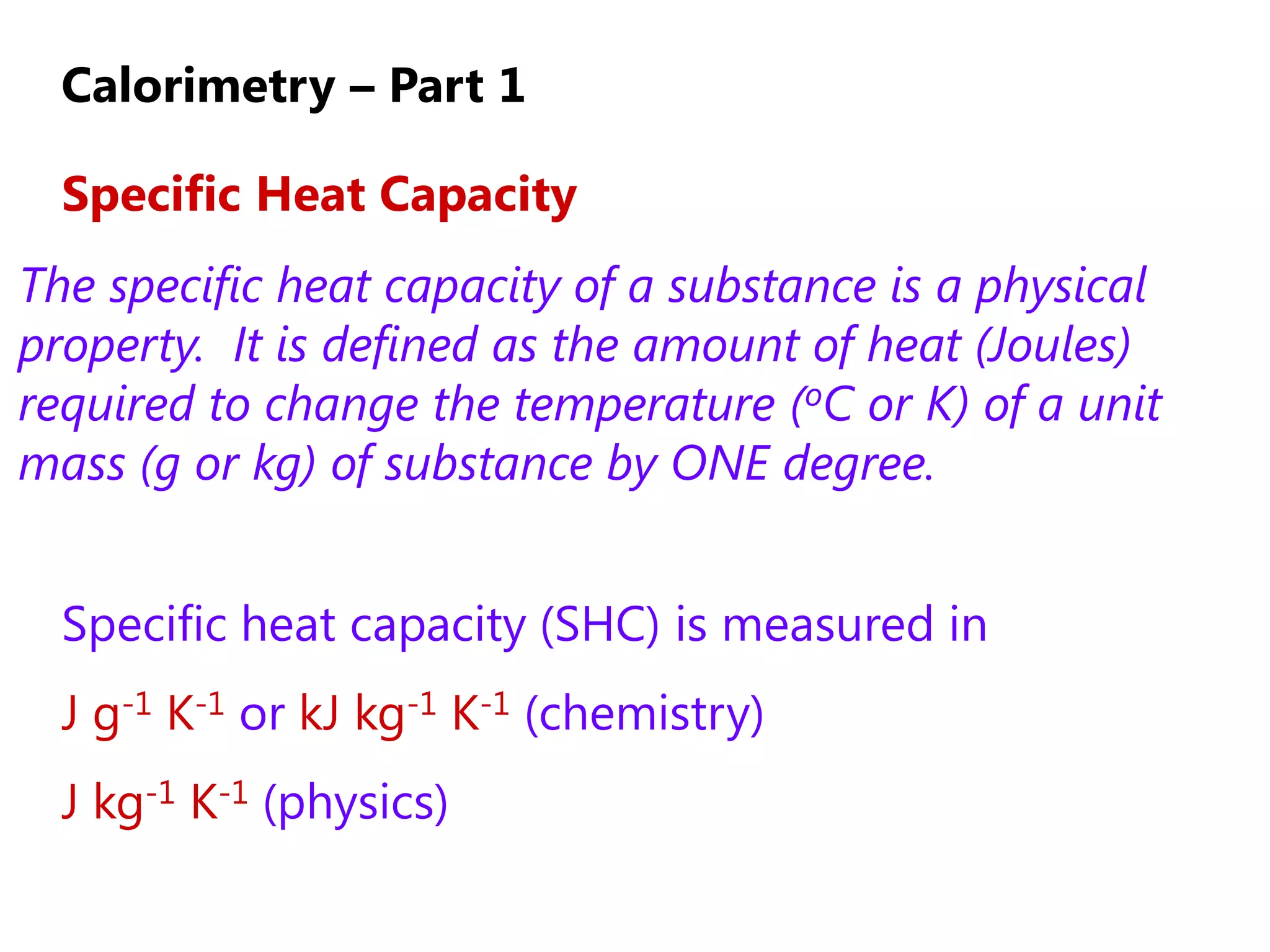
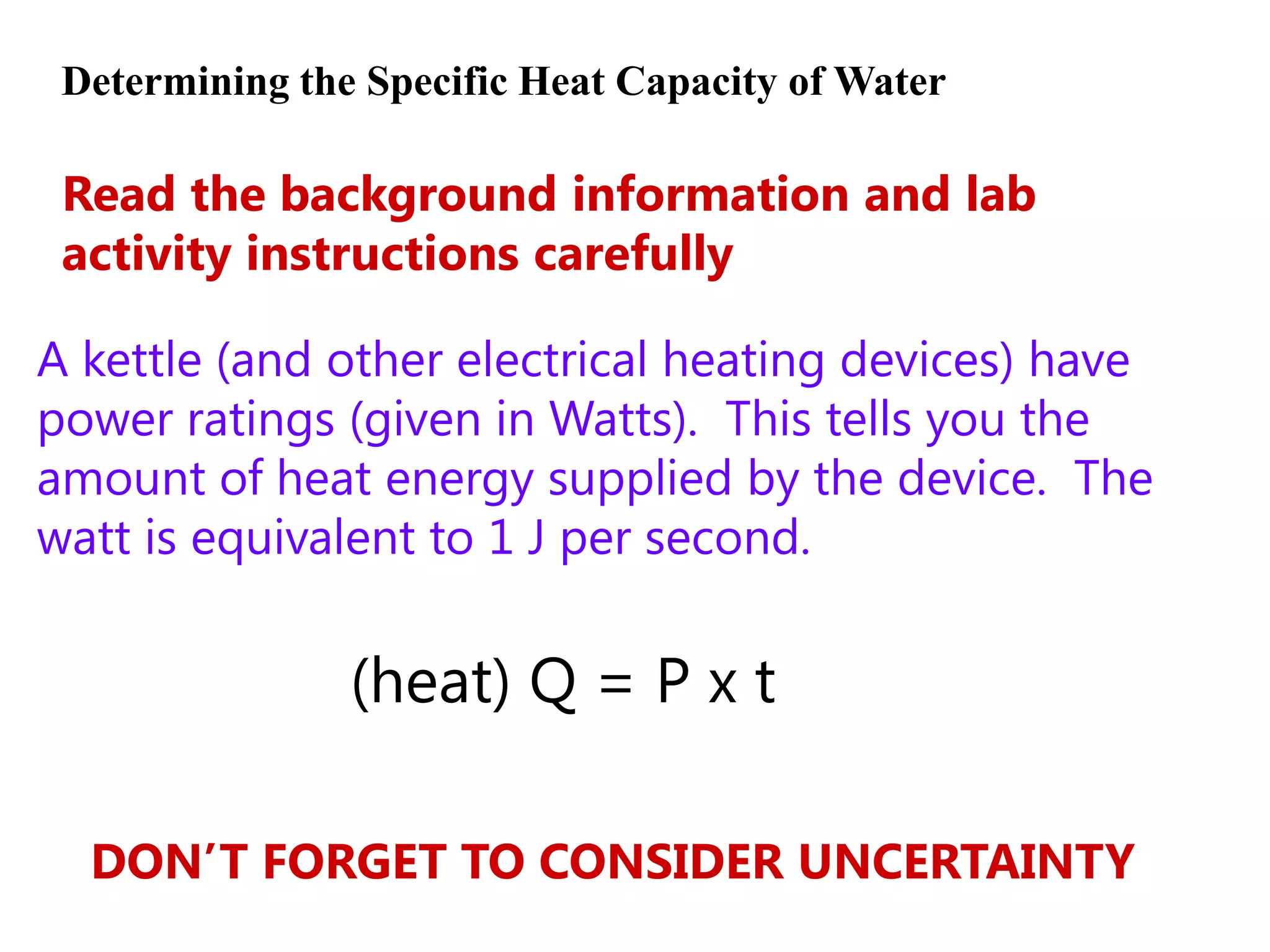
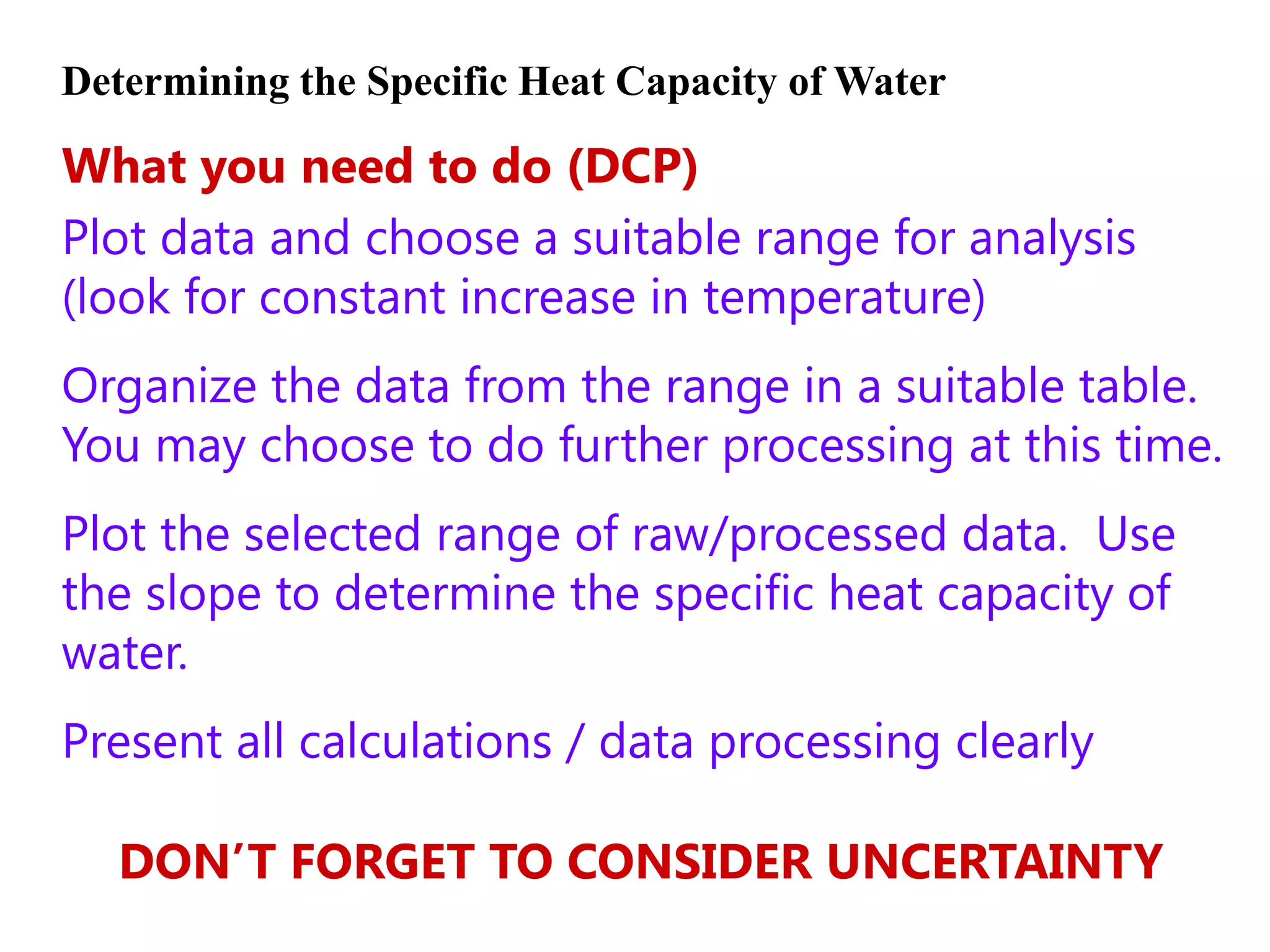
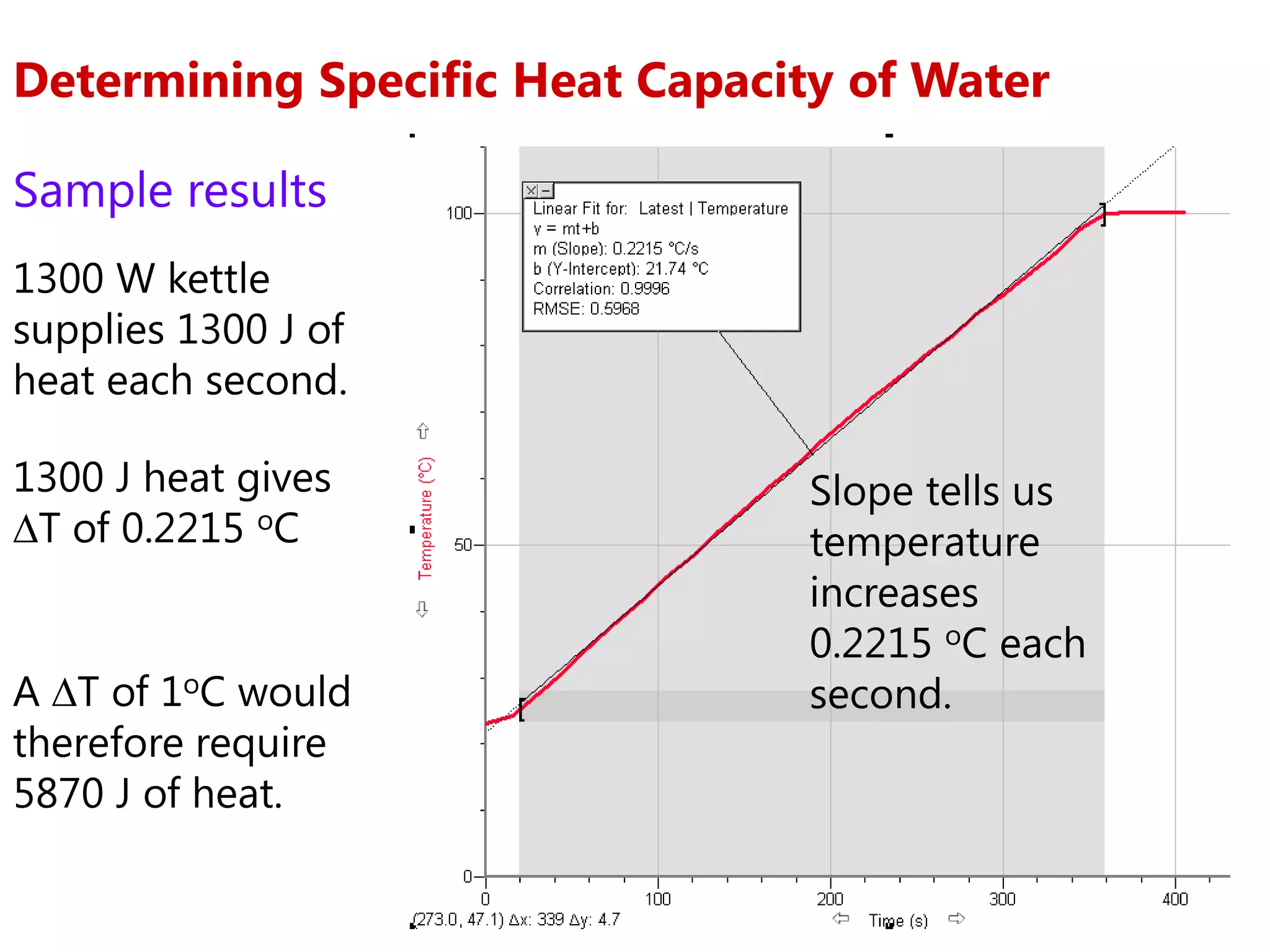
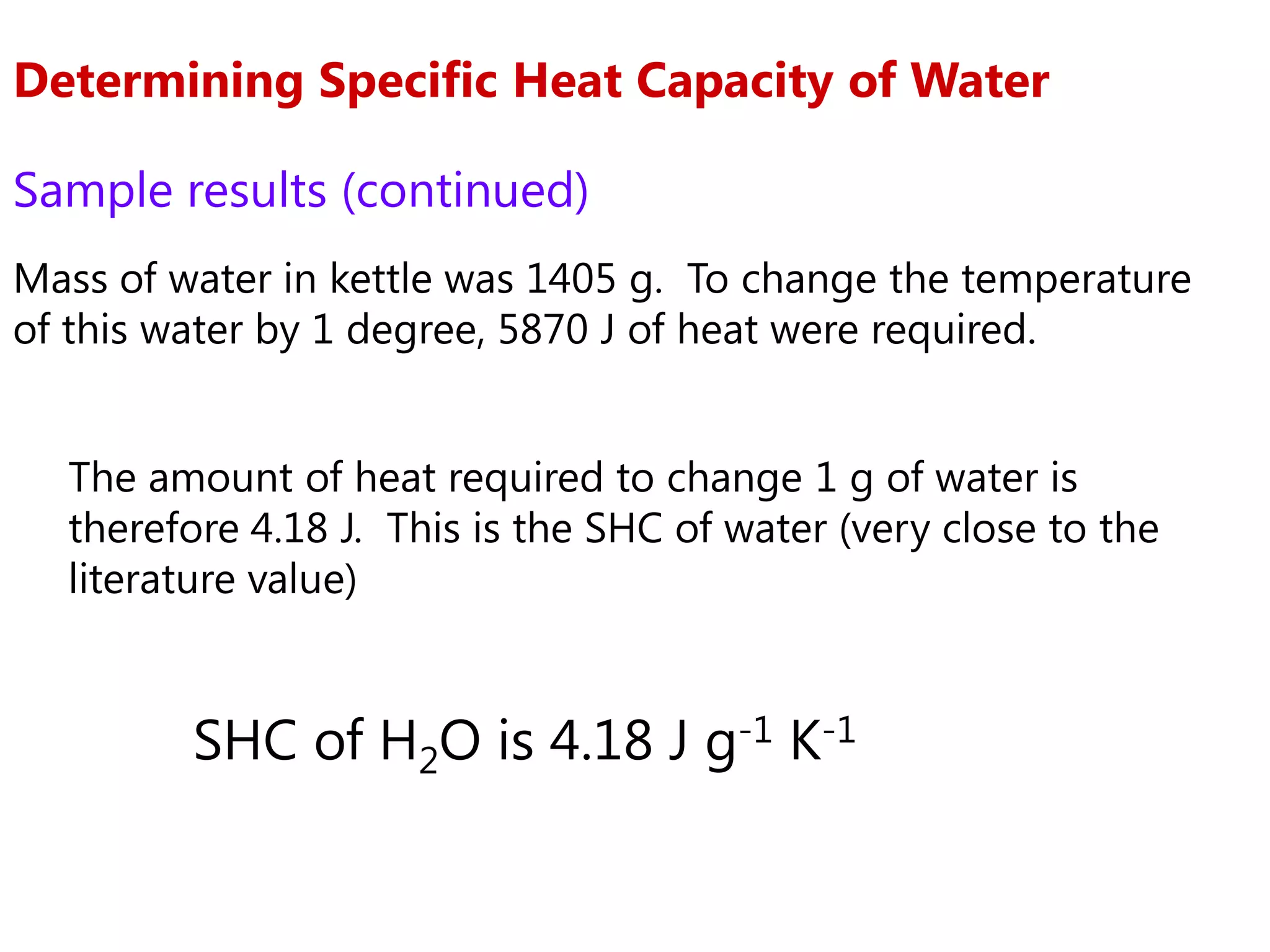
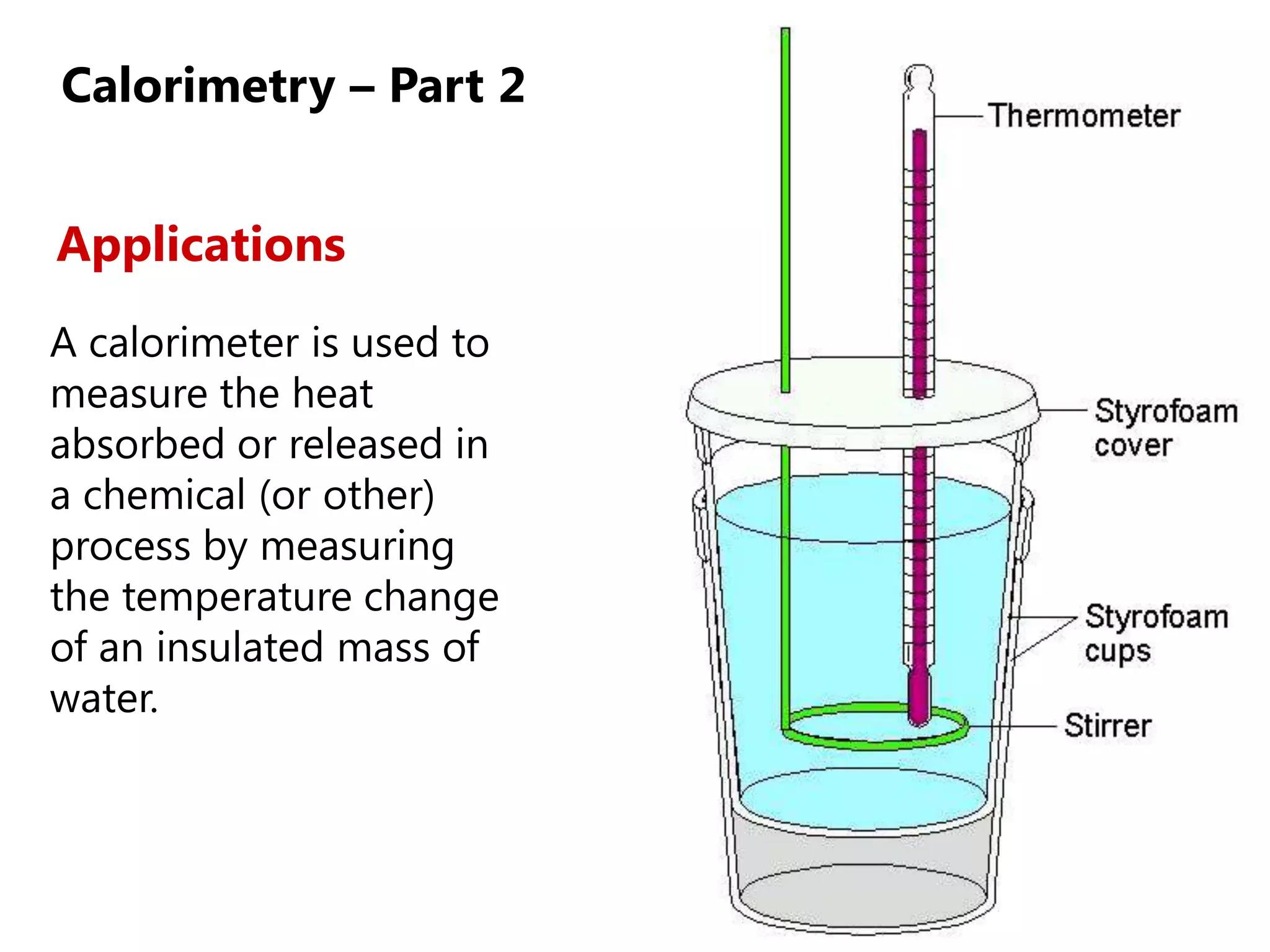
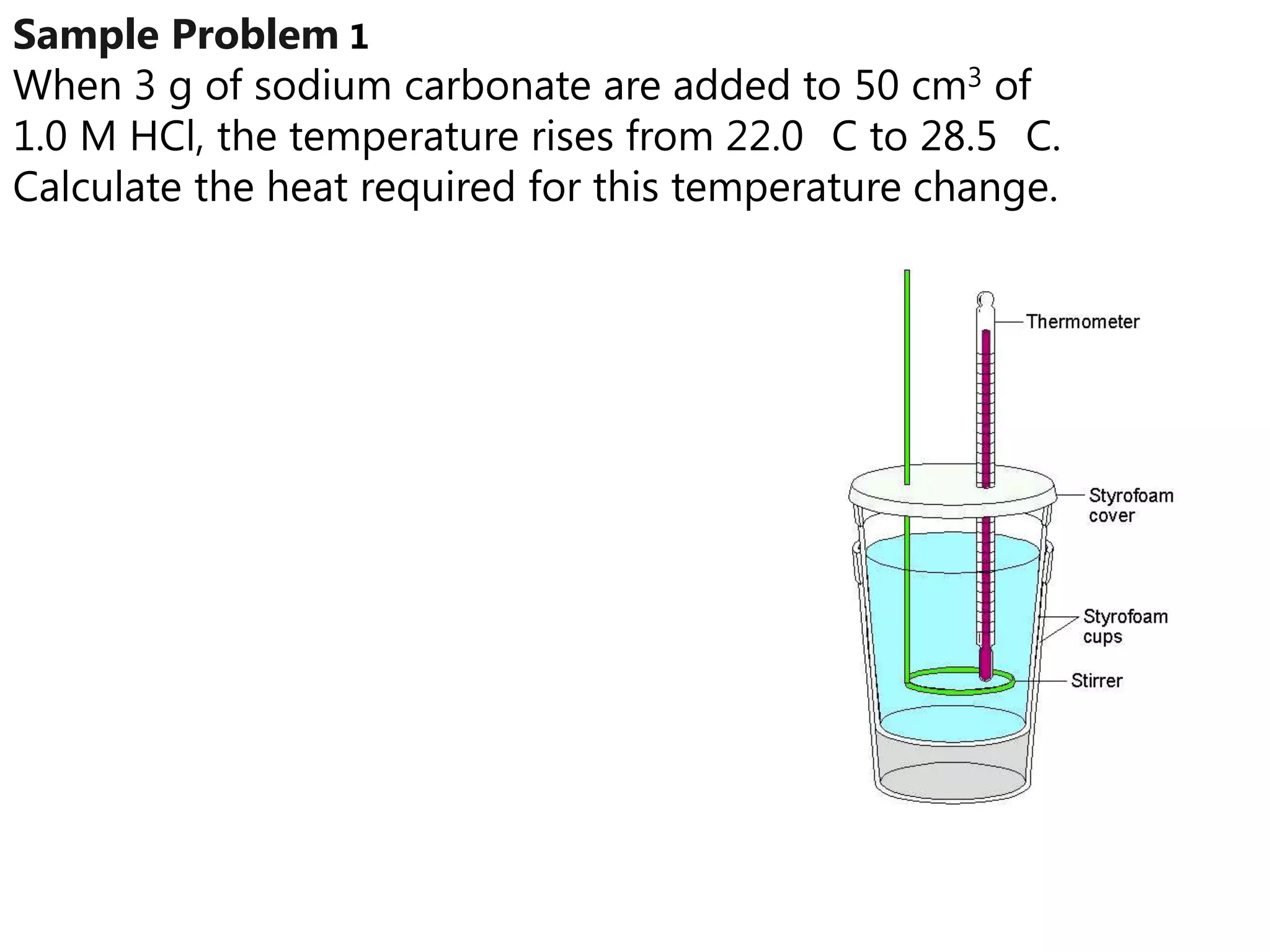
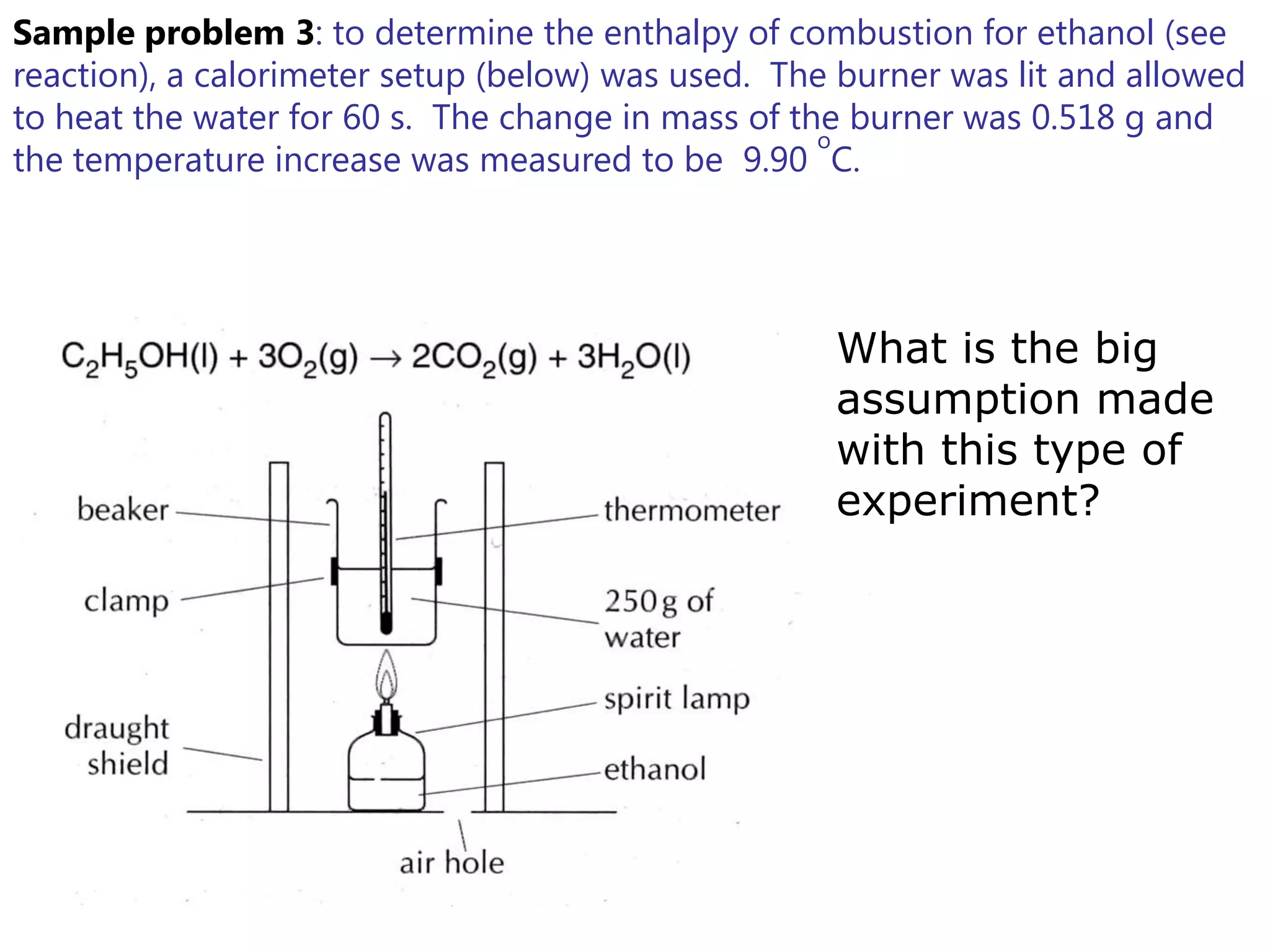
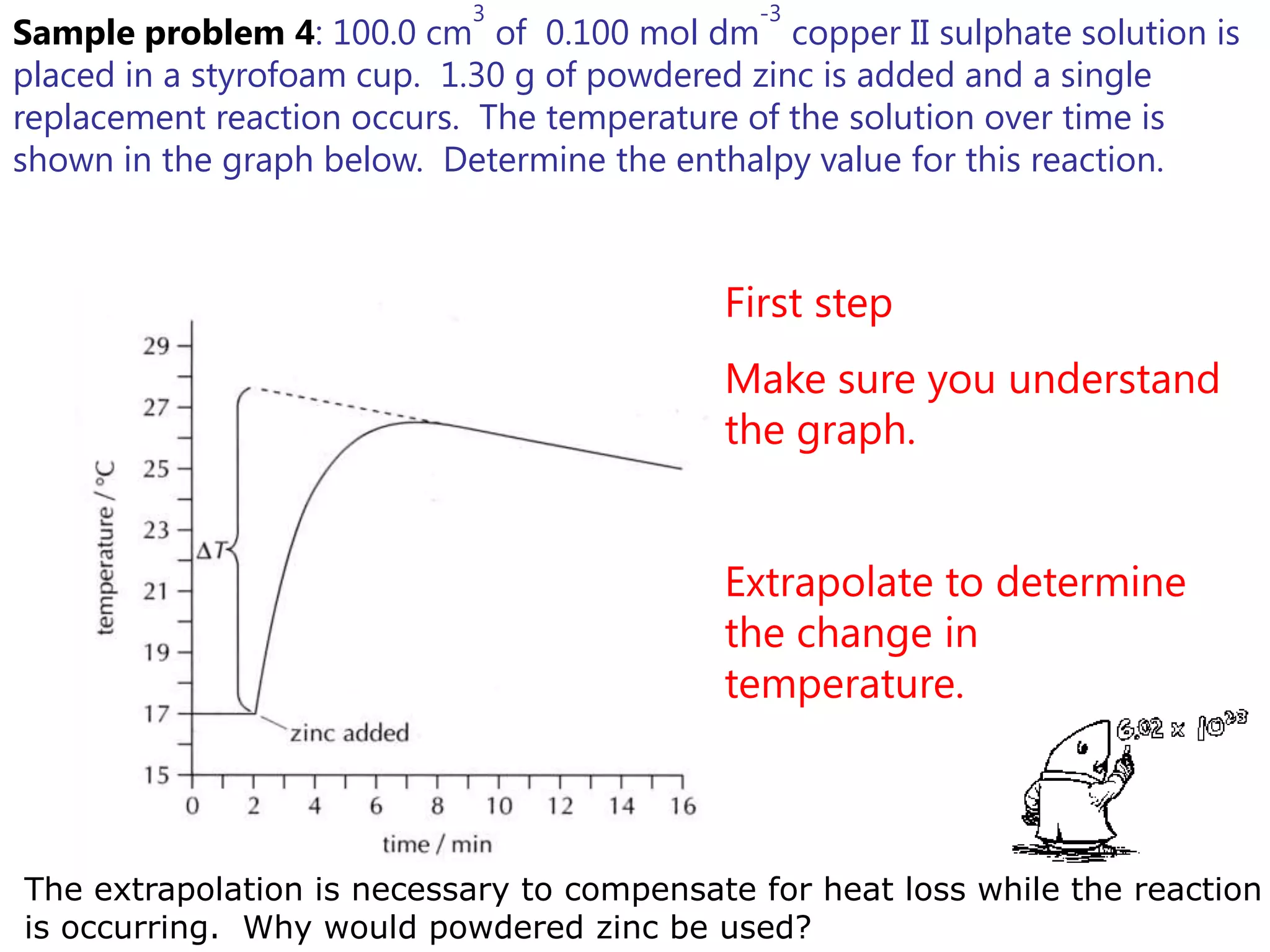
2) Calorimetry can be used to determine enthalpy changes by measuring the temperature change of a reaction mixture in a calorimeter.
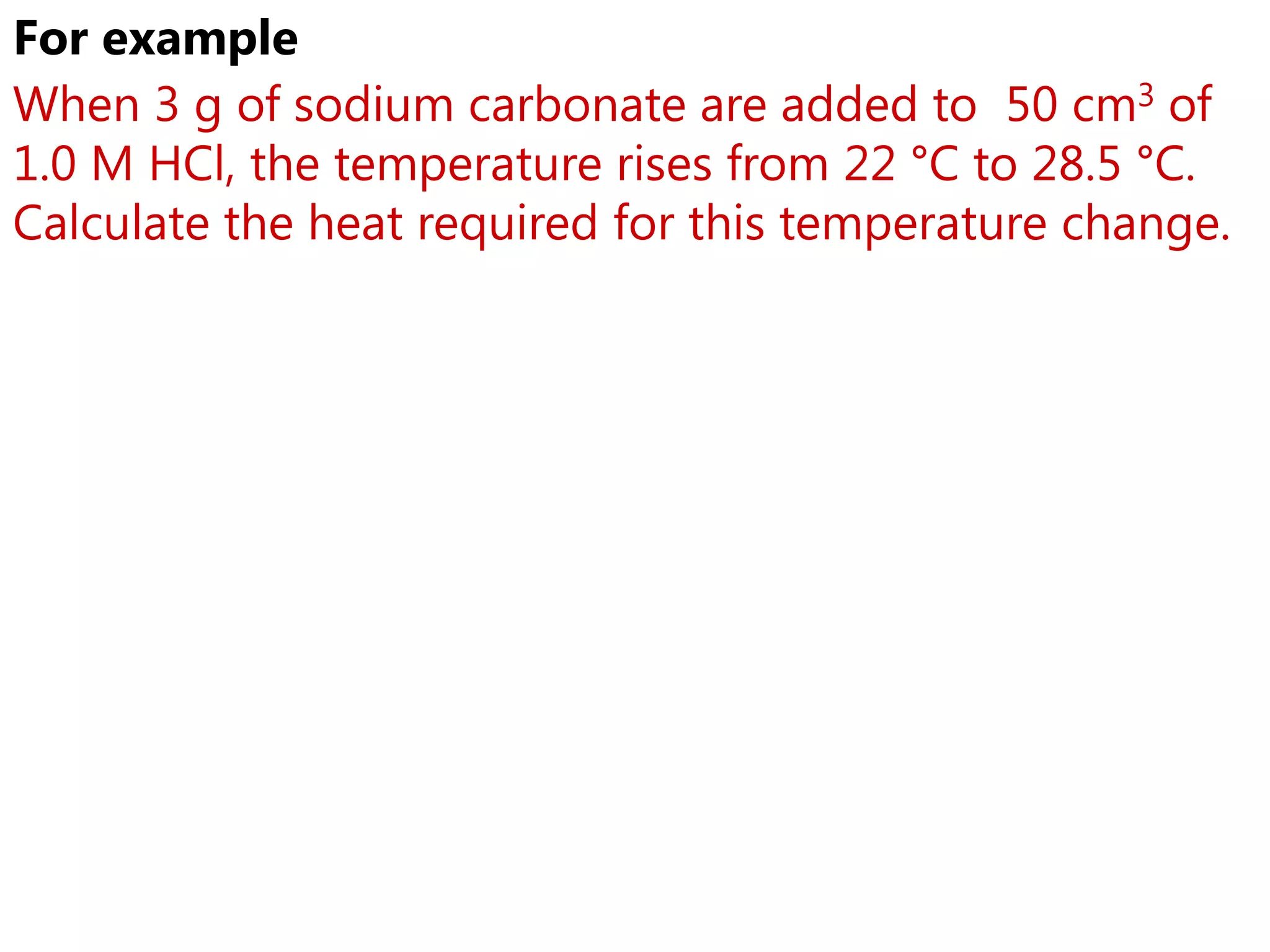
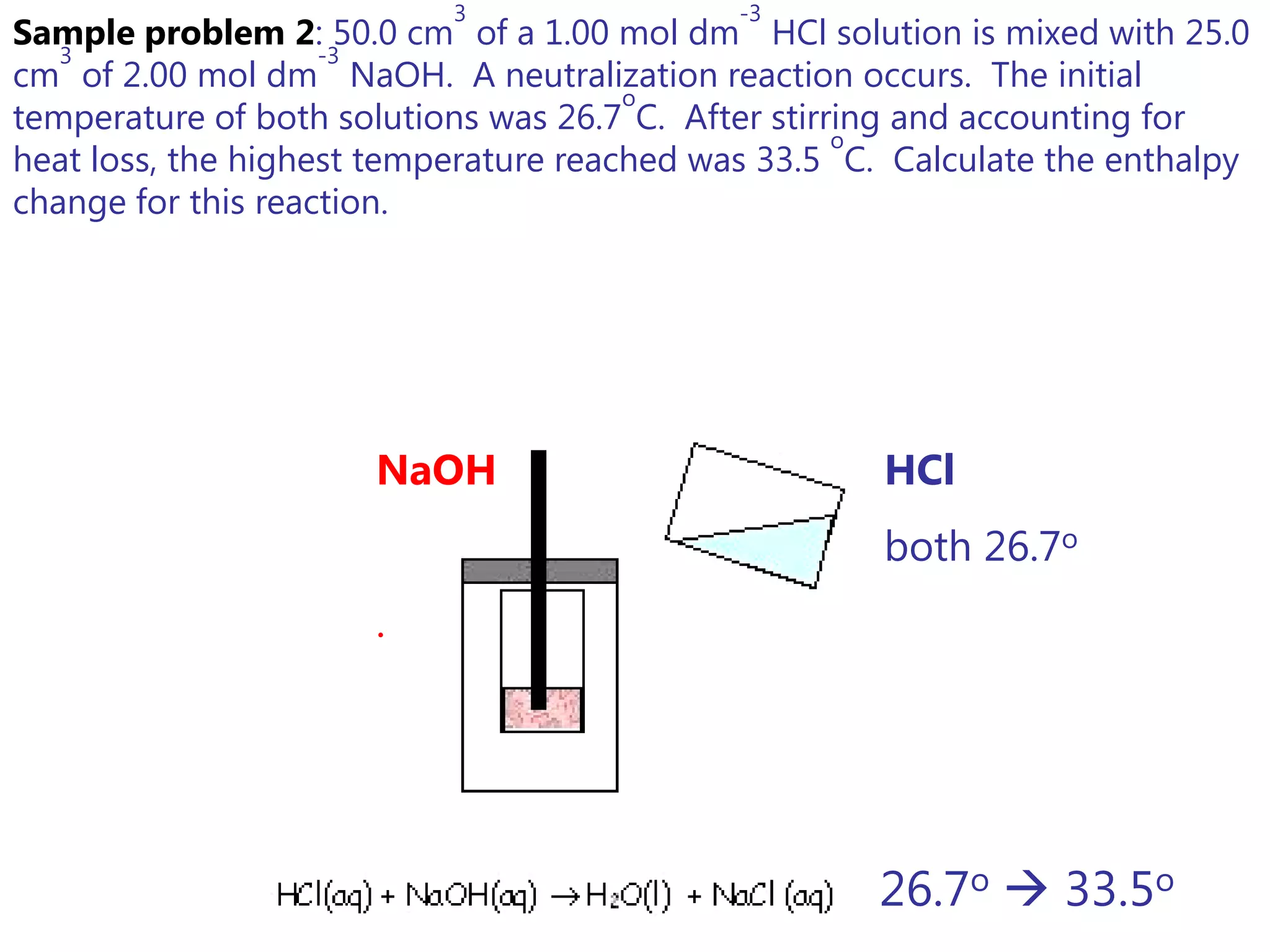
3) Sample problems demonstrate how to use calorimetry data like temperature changes, masses, and concentrations to calculate enthalpy changes for chemical reactions.