This document provides an overview of chemical thermodynamics, including:
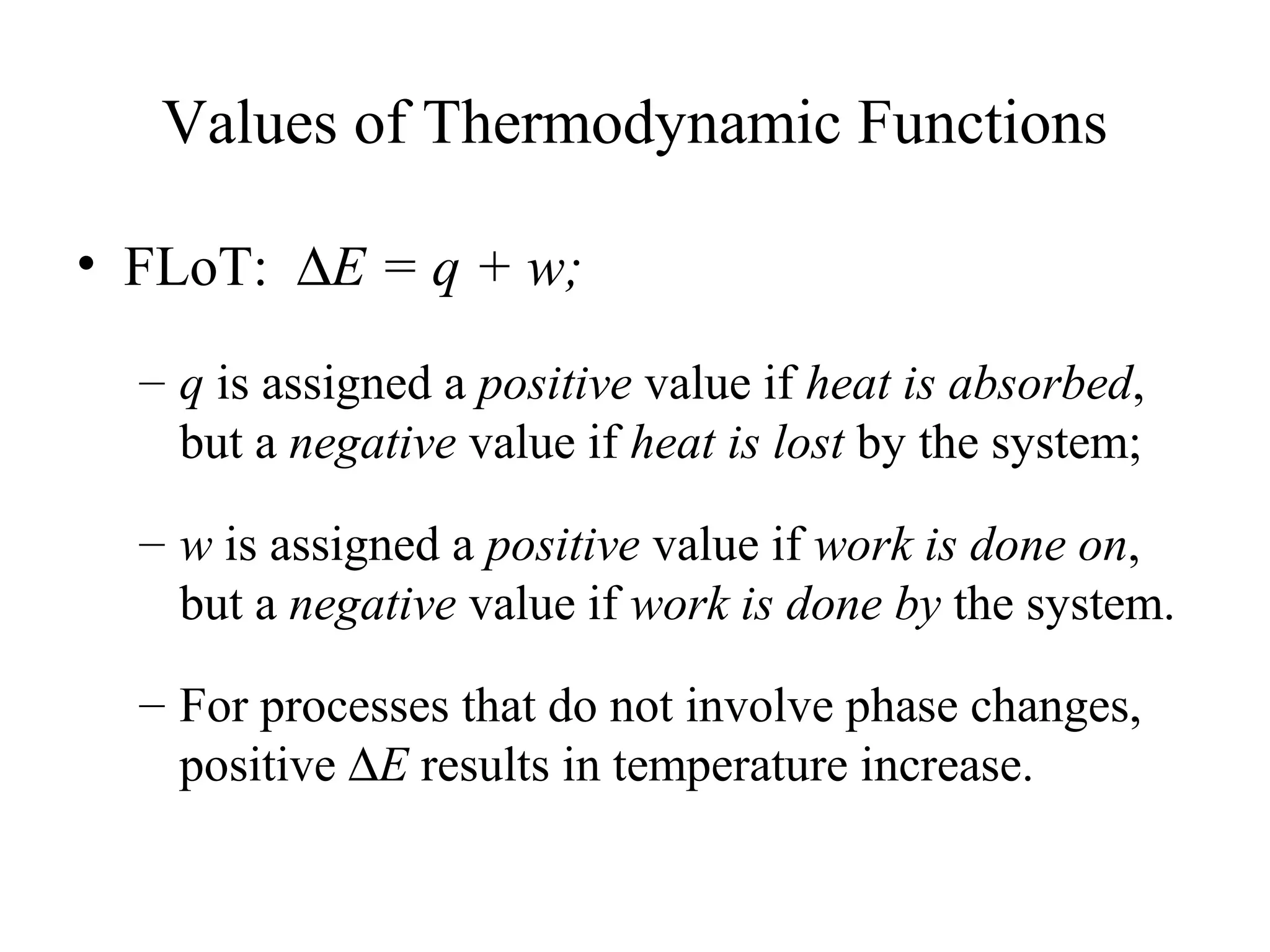
- The first law of thermodynamics which states that change in internal energy equals heat added plus work done.
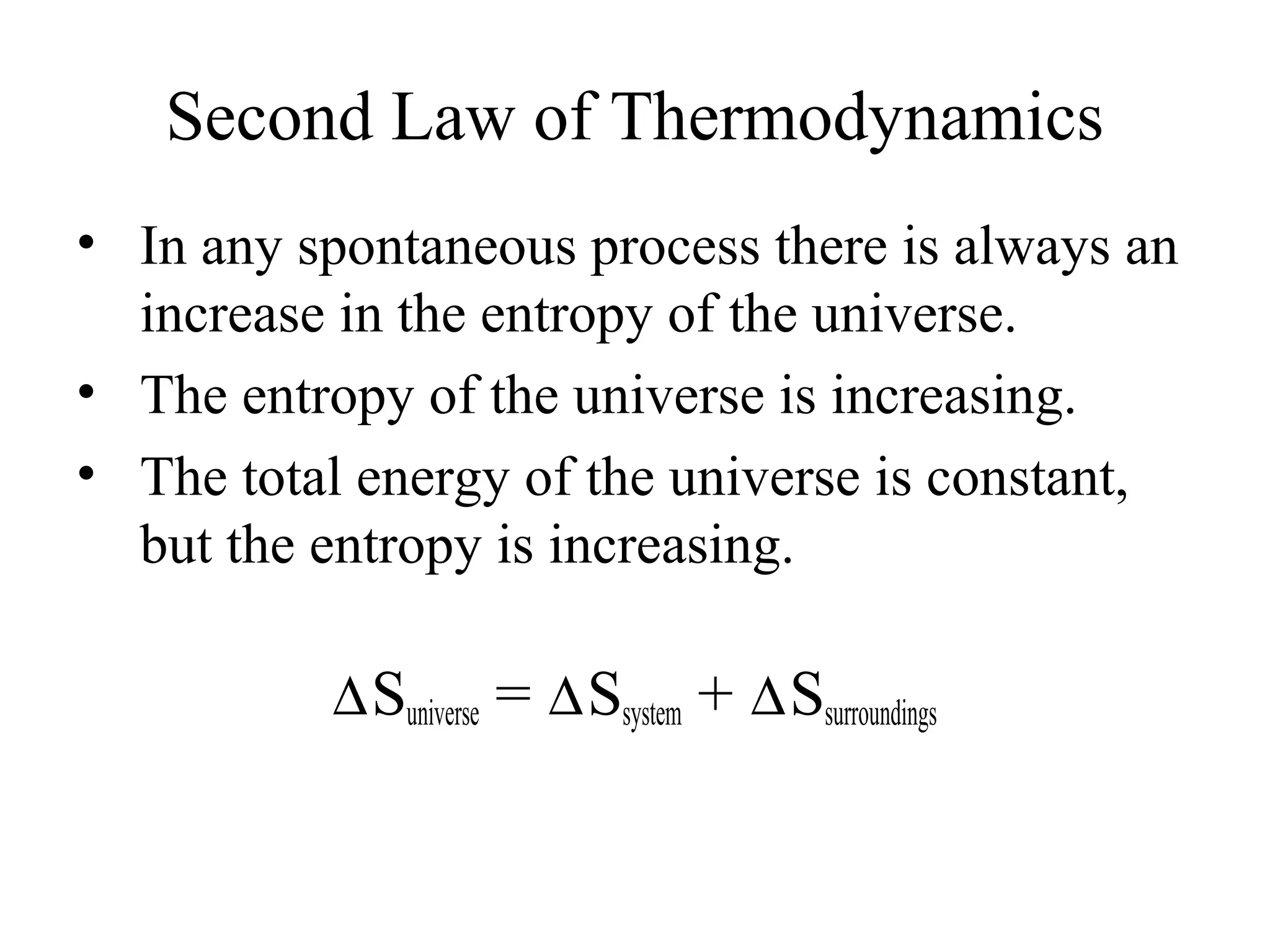
- The second law of thermodynamics which states that the entropy of the universe increases for spontaneous processes.
- How changes in entropy and free energy determine whether processes are spontaneous, with spontaneous processes favoring higher entropy and more negative free energy.