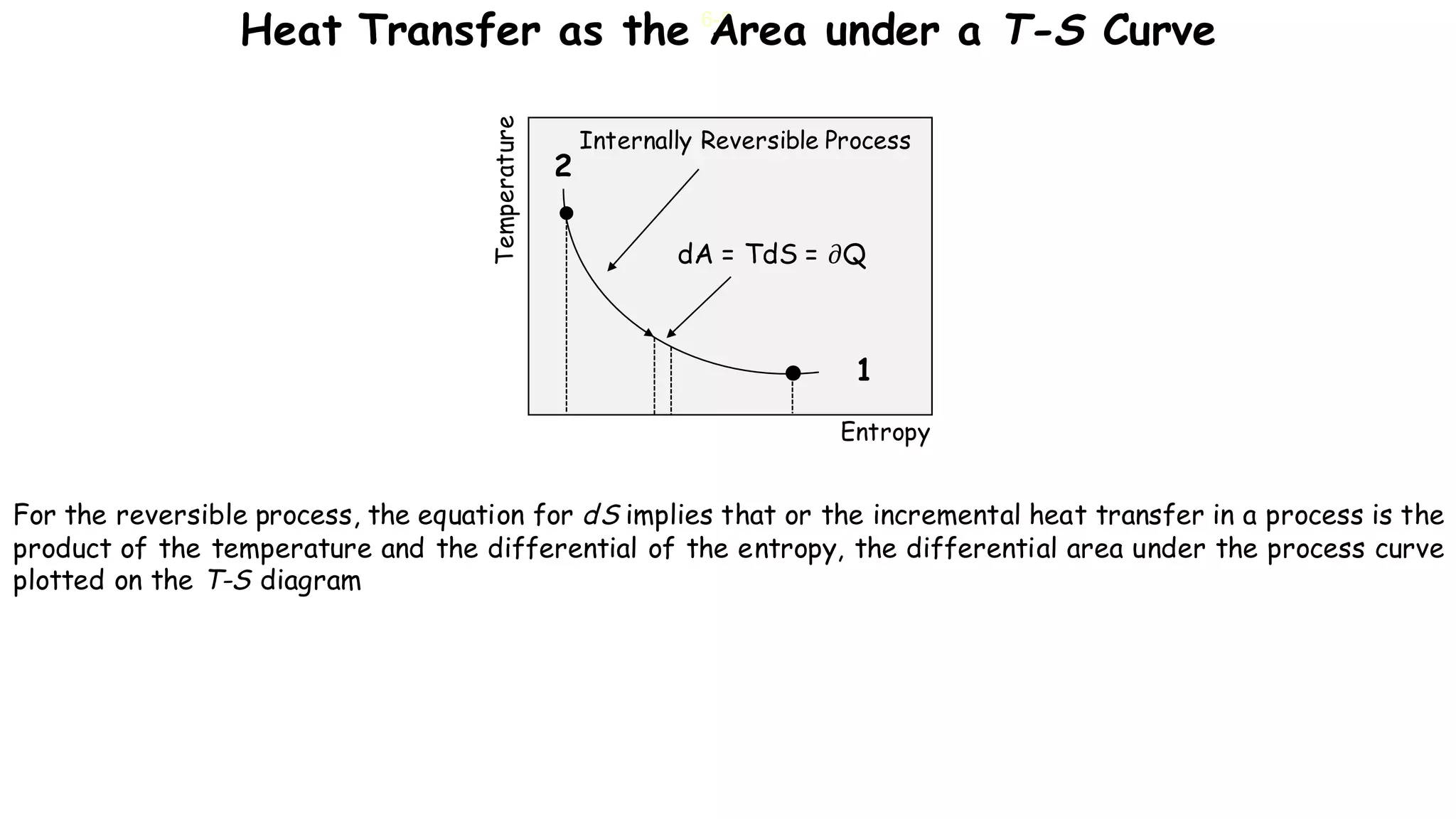
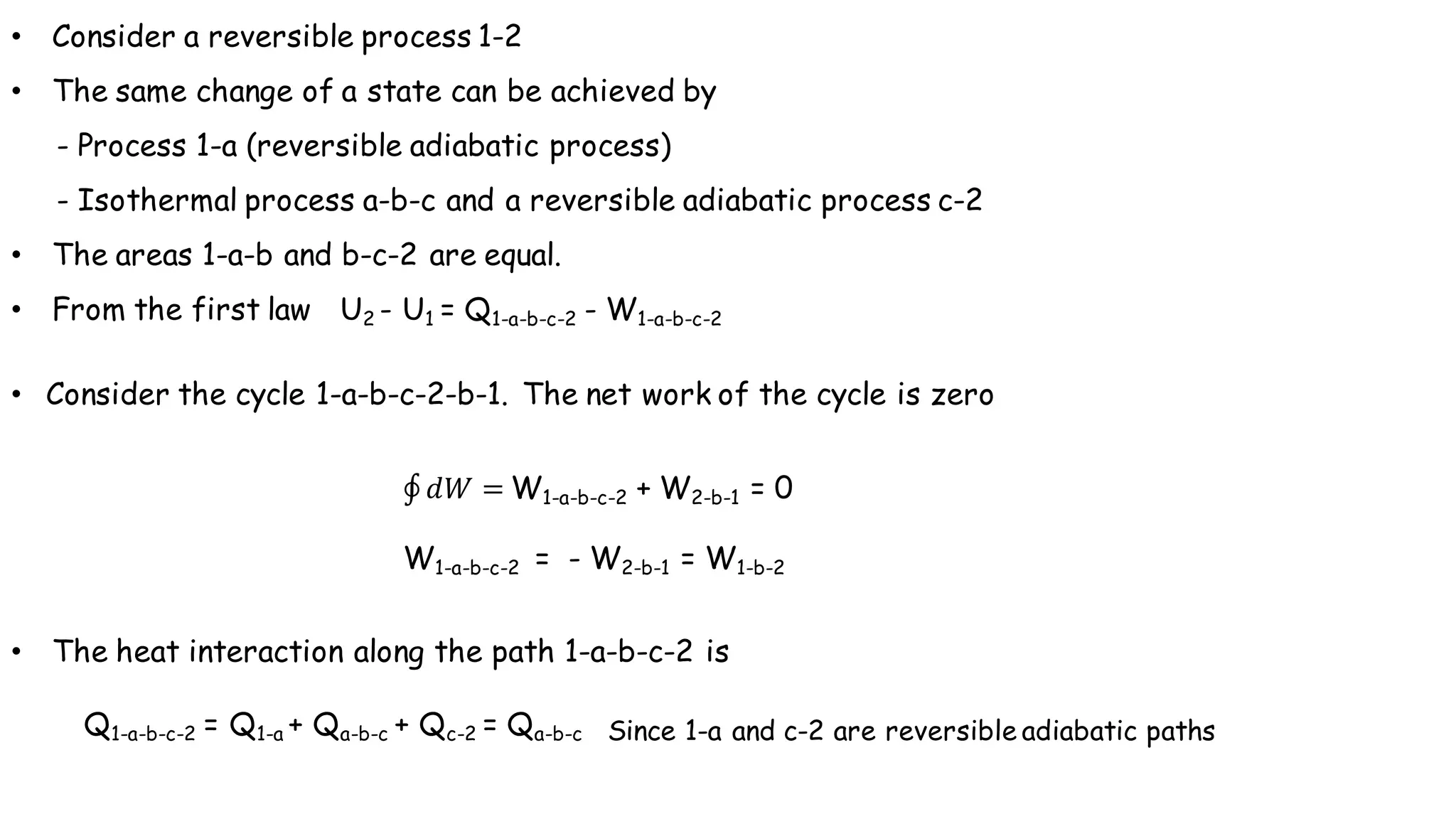
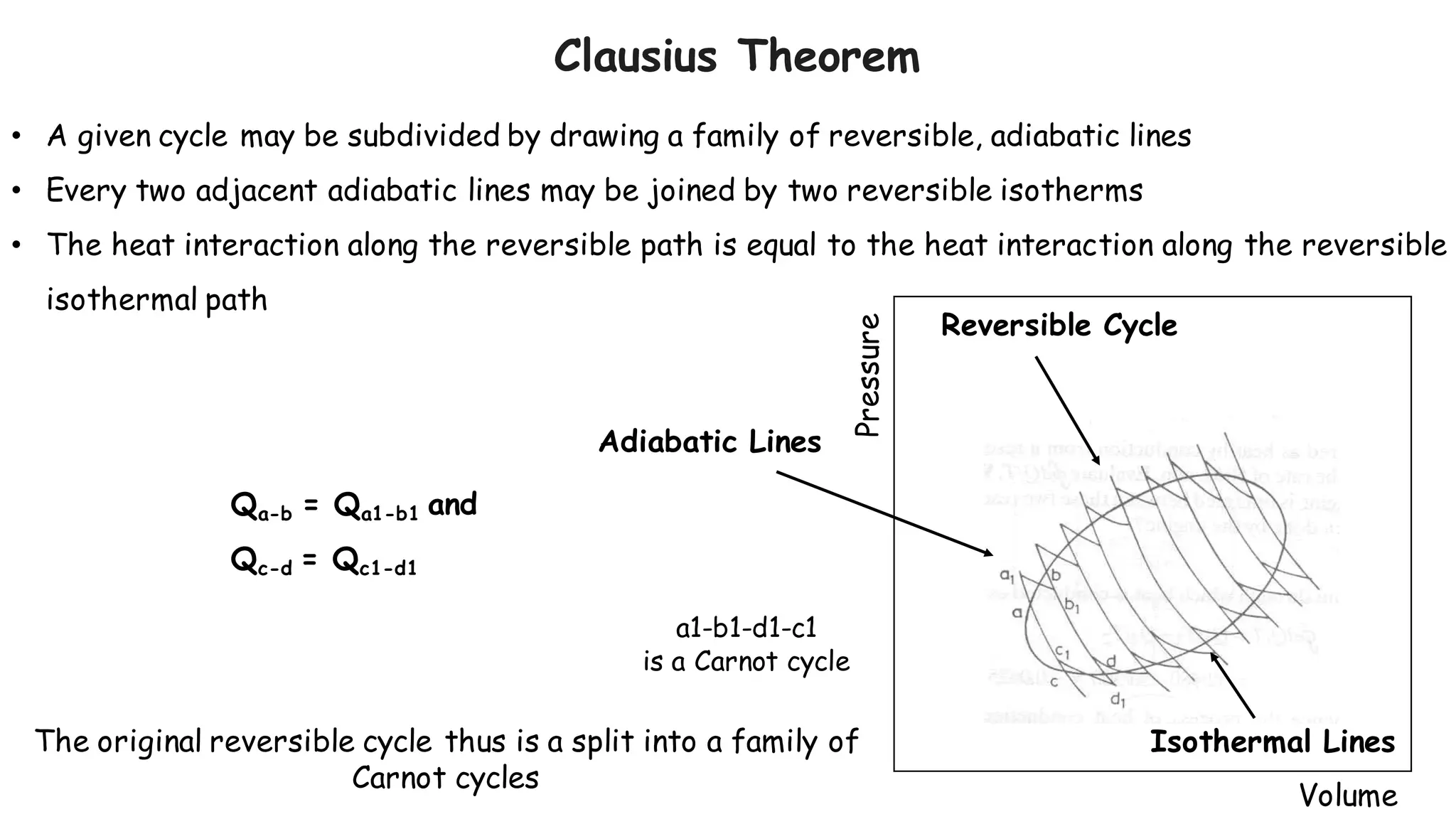
- Any reversible process can be approximated by a series of reversible, isothermal and reversible, adiabatic processes connected by intermediate states.
- The heat interaction along the reversible path is equal to the heat interaction along the reversible isothermal path between the same initial and final states.
- Therefore, a reversible process can be replaced by a zig-zag path consisting of reversible adiabatic and isothermal processes, satisfying the first law of thermodynamics.
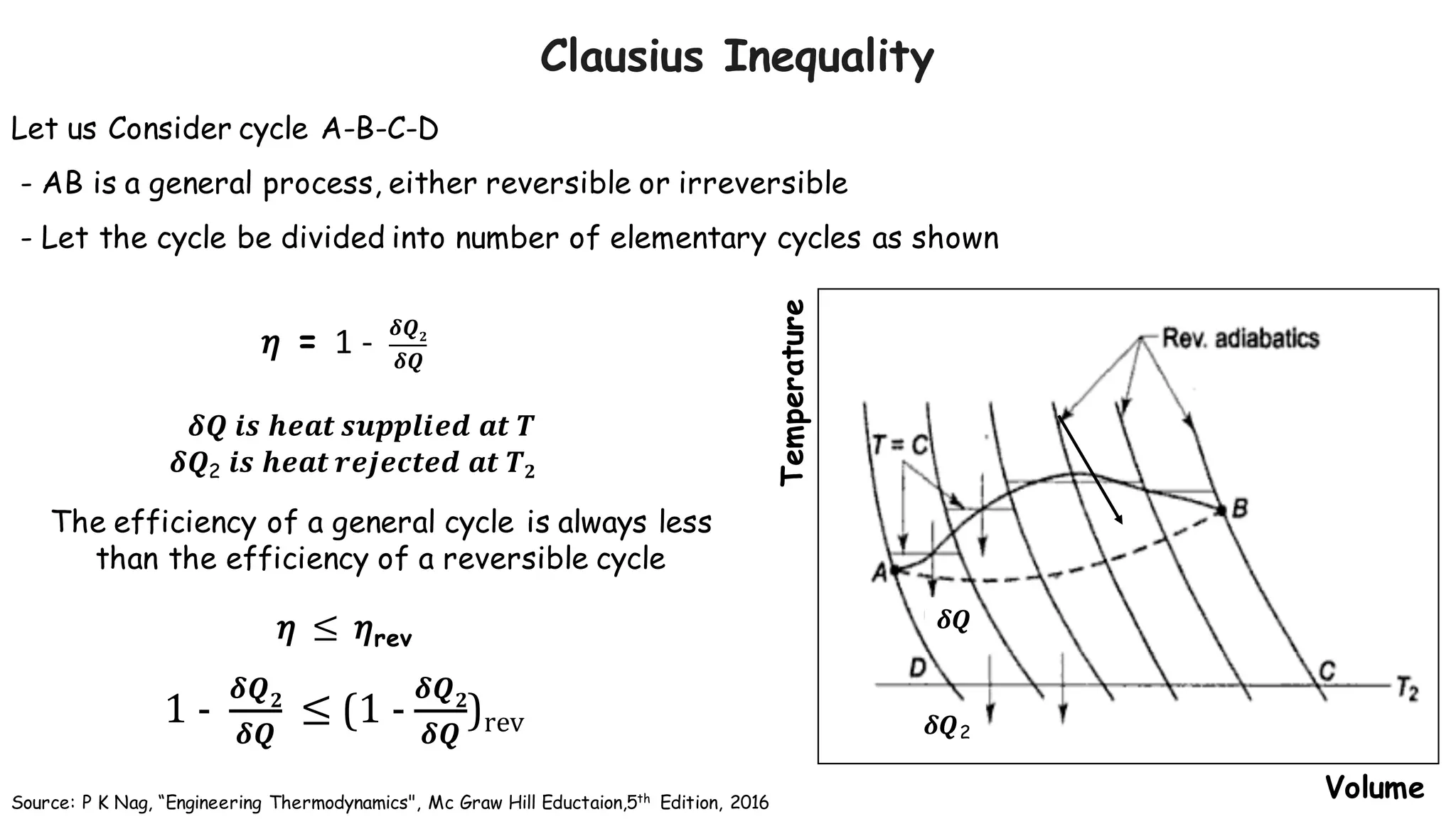
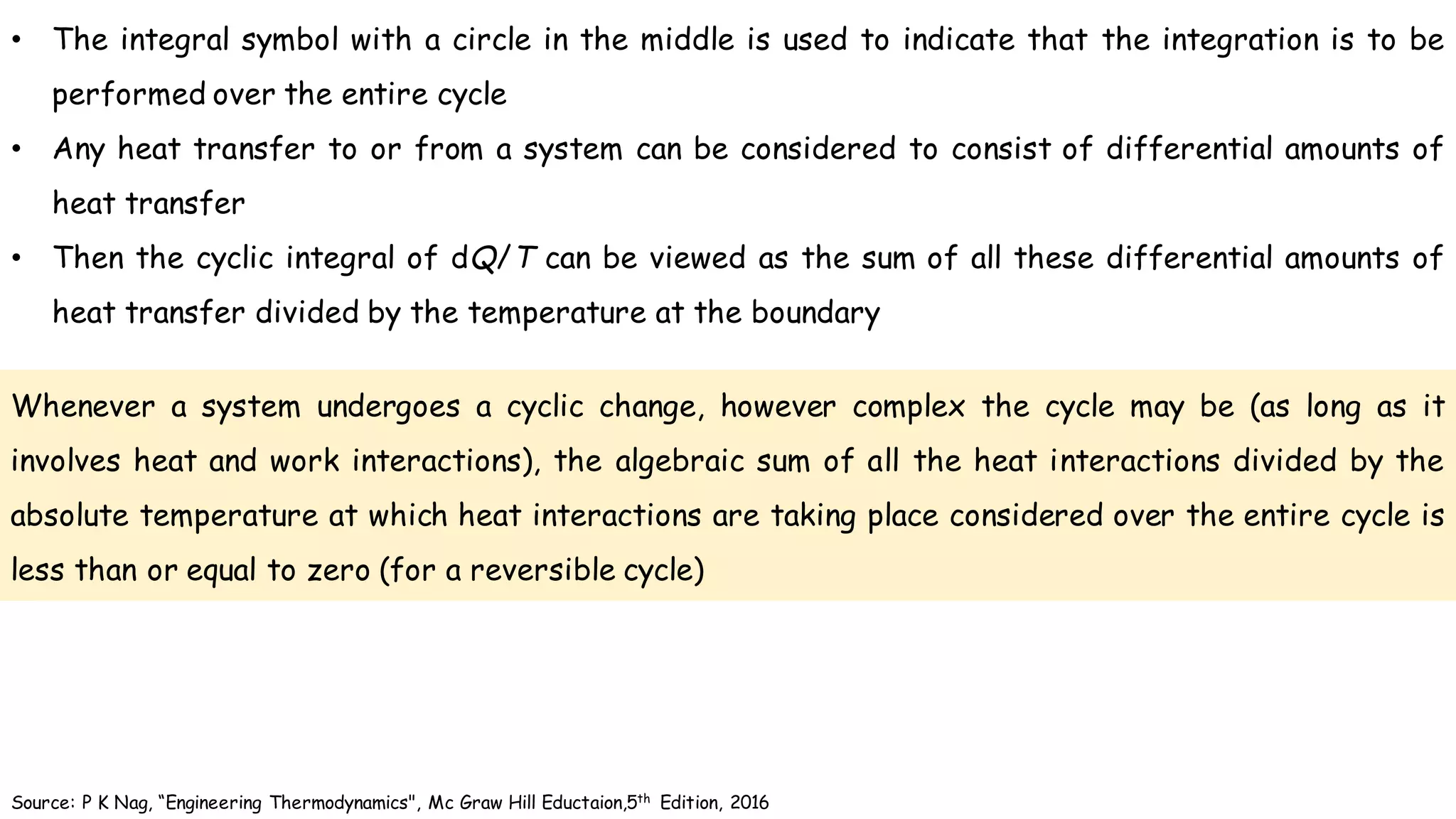
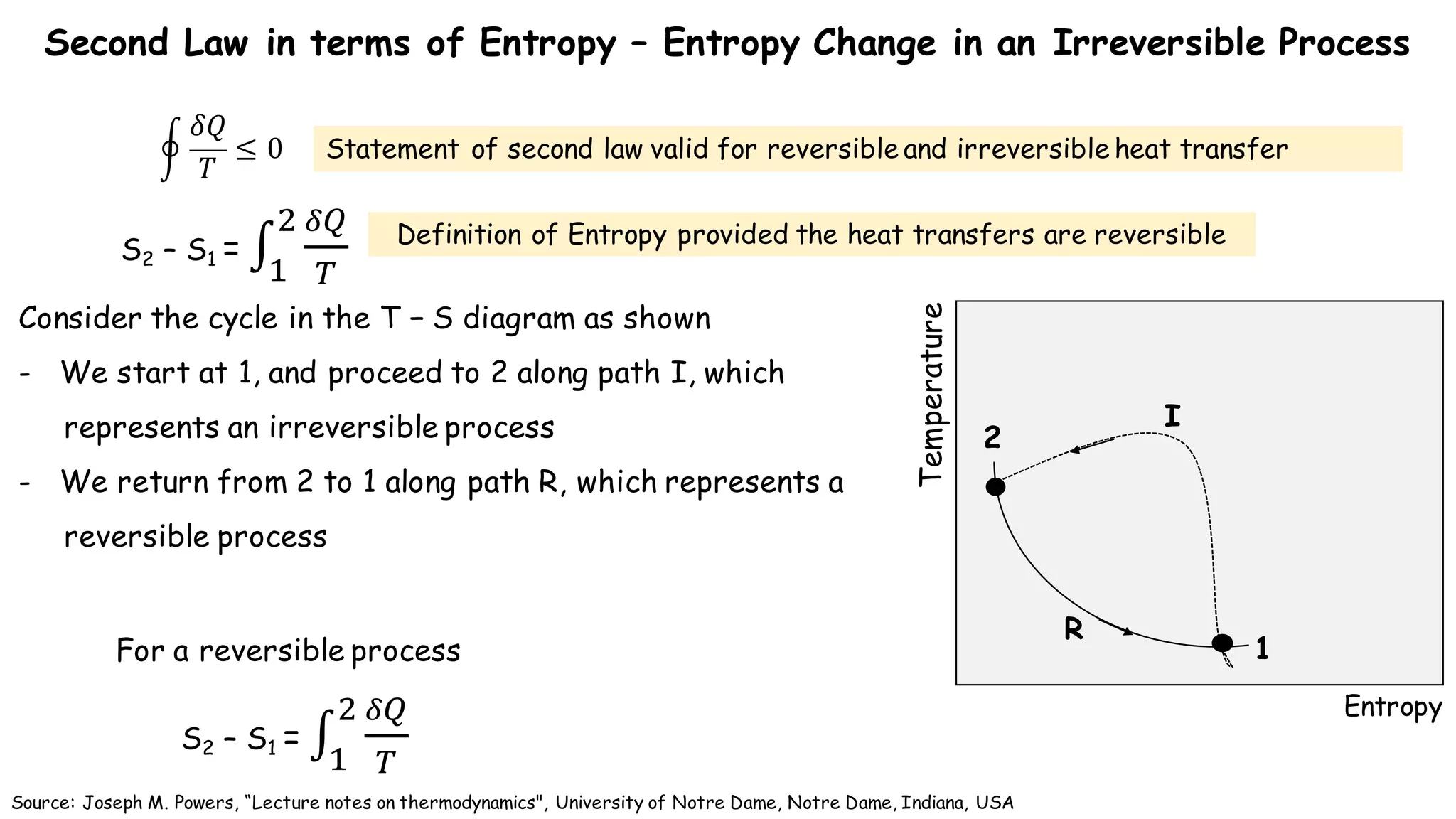
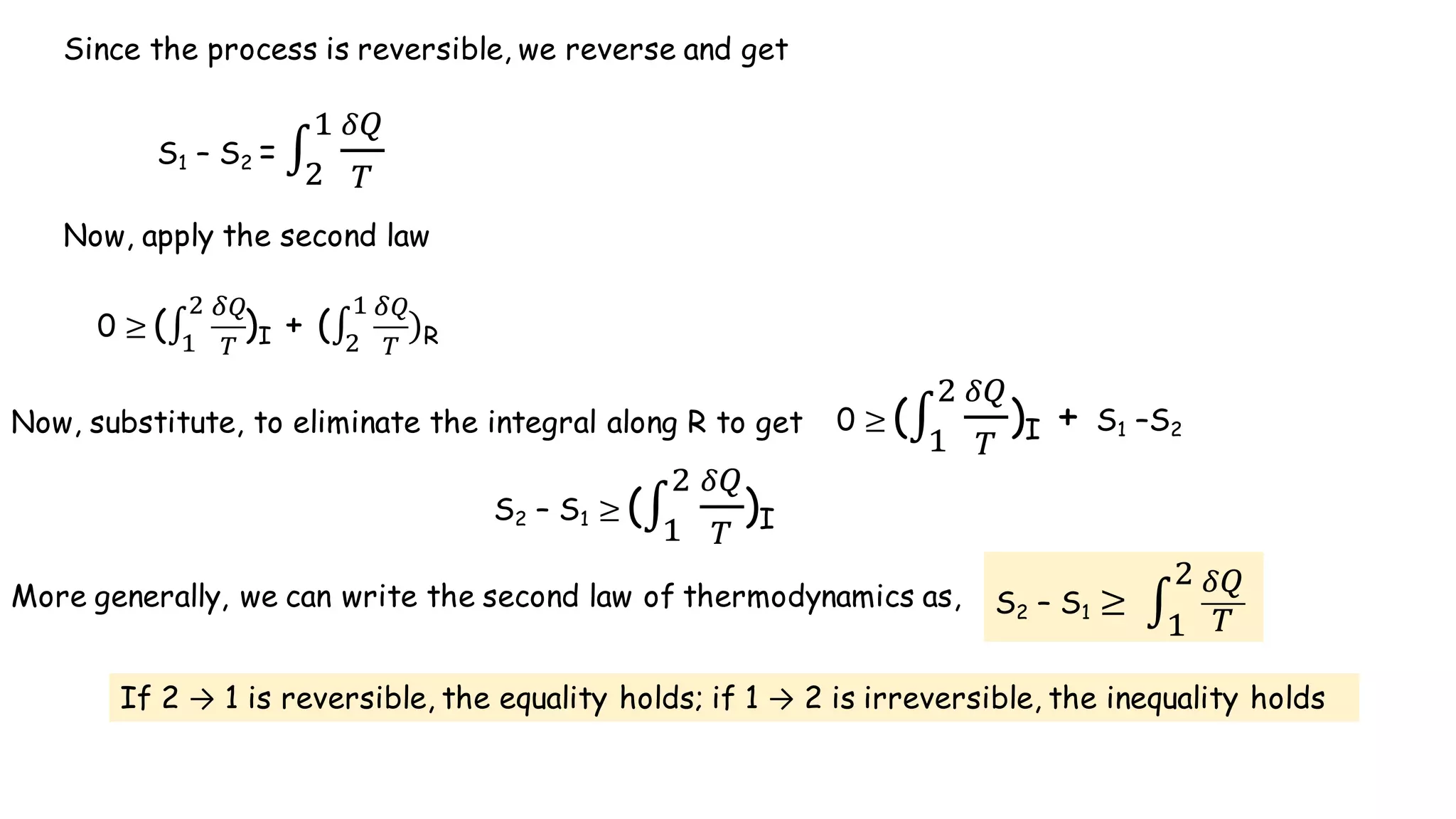
- According to the Clausius theorem, the integral of heat transfer divided by temperature around any cyclic process is equal to zero for a reversible process. This leads to the definition of entropy as a state function.











![scale by the constant mass m to get the
corresponding intensive variable s = S/m
Integrating
Differential Form
This is the heat transfer equivalent to
s2 – s1= ∫
Q'
)
+
(
𝛿𝑠 =
𝛿𝑞
𝑇
𝛿𝑞 = 𝑇. 𝛿𝑠
] 𝛿𝑞
+
(
= ] 𝑇. 𝛿𝑠
+
(
1q2 = ∫ 𝑇. 𝛿𝑠
+
(
1w2= ∫ 𝑇. 𝛿𝑠
+
(
Source: Joseph M. Powers, “Lecture notes on thermodynamics", University of Notre Dame, Notre Dame, Indiana, USA](https://image.slidesharecdn.com/entropy-170118101122/75/Entropy-17-2048.jpg)






![6-3
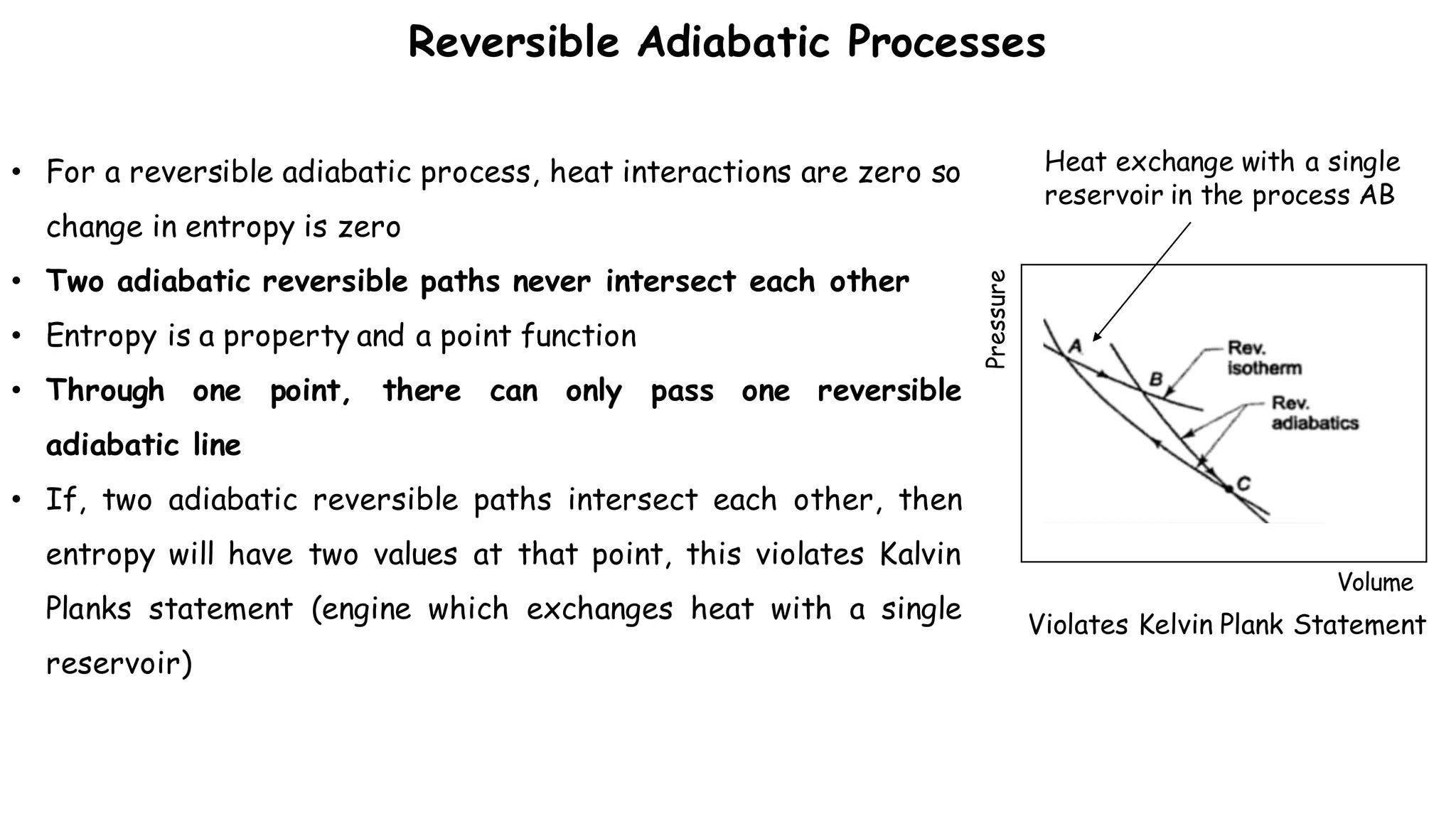
The entropy change of an isolated system is the sum of the entropy changes of its components, and
is never less than zero
Entropy Change of an Isolated System
“The entropy of an isolated system either increases or, in the limit, remains constant.”
The principle of entropy increase: The entropy of an isolated system can never decrease. It always
increases with every irreversible process and remains constant, only when the process is reversible.
dSsys =
`Q'
)TcT
dSsur =
Q'
)TdR
dSiso = dSsys + dSsur
= 𝛿𝑄 [
(
)TdR
-
(
)TcT
] > 0
The entropy of an isolated system either increases if it undergoes an irreversible process
System
Tsys
Surroundings
Tsur
SQ
If, Tsys = Tsur, the process would occur reversibly and entropy will remain constant
Source: D. S. Kumar, “Engineering Thermodynamics", S.K. Kataria & Sons, 2nd Edition](https://image.slidesharecdn.com/entropy-170118101122/75/Entropy-26-2048.jpg)