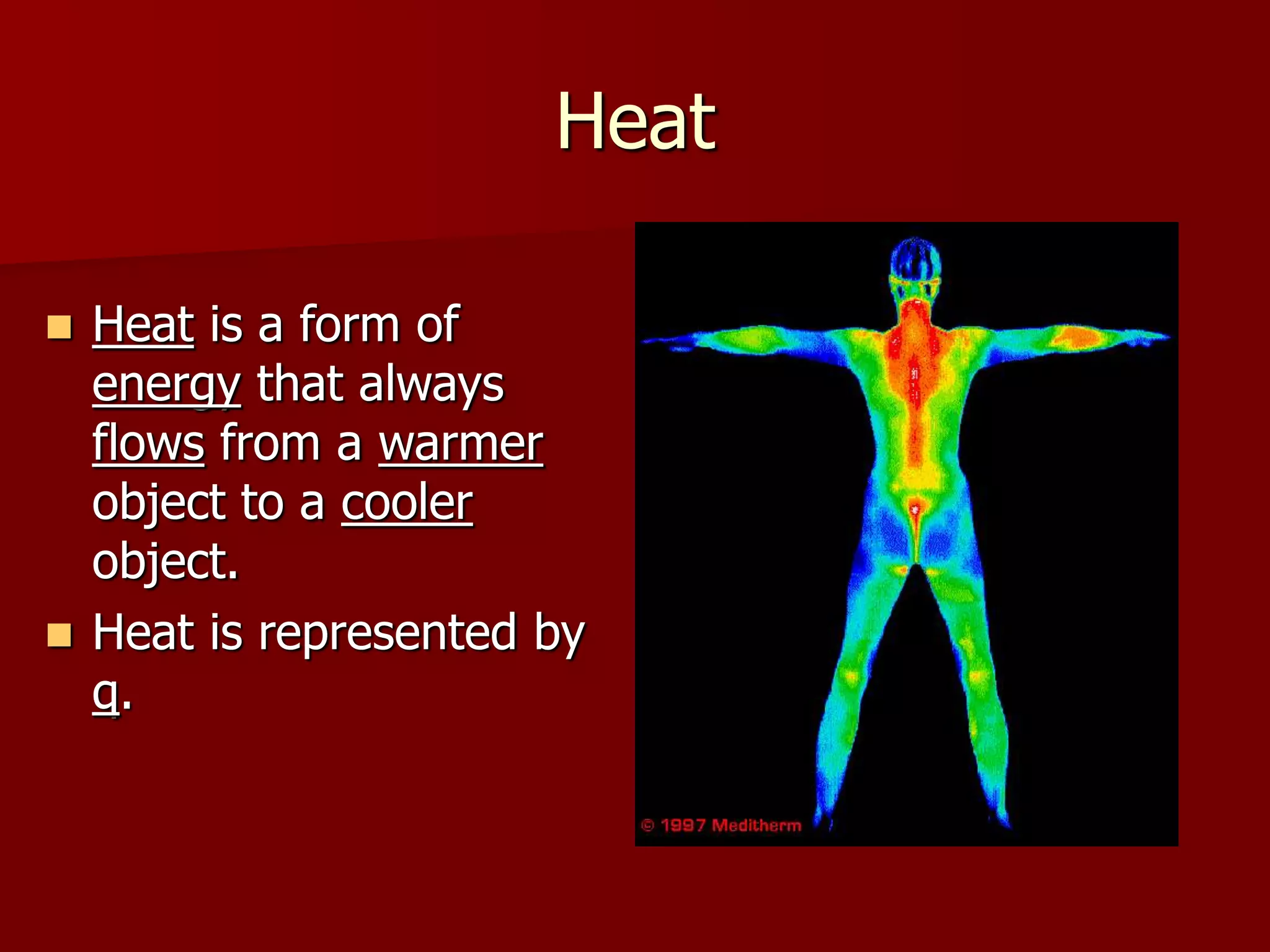
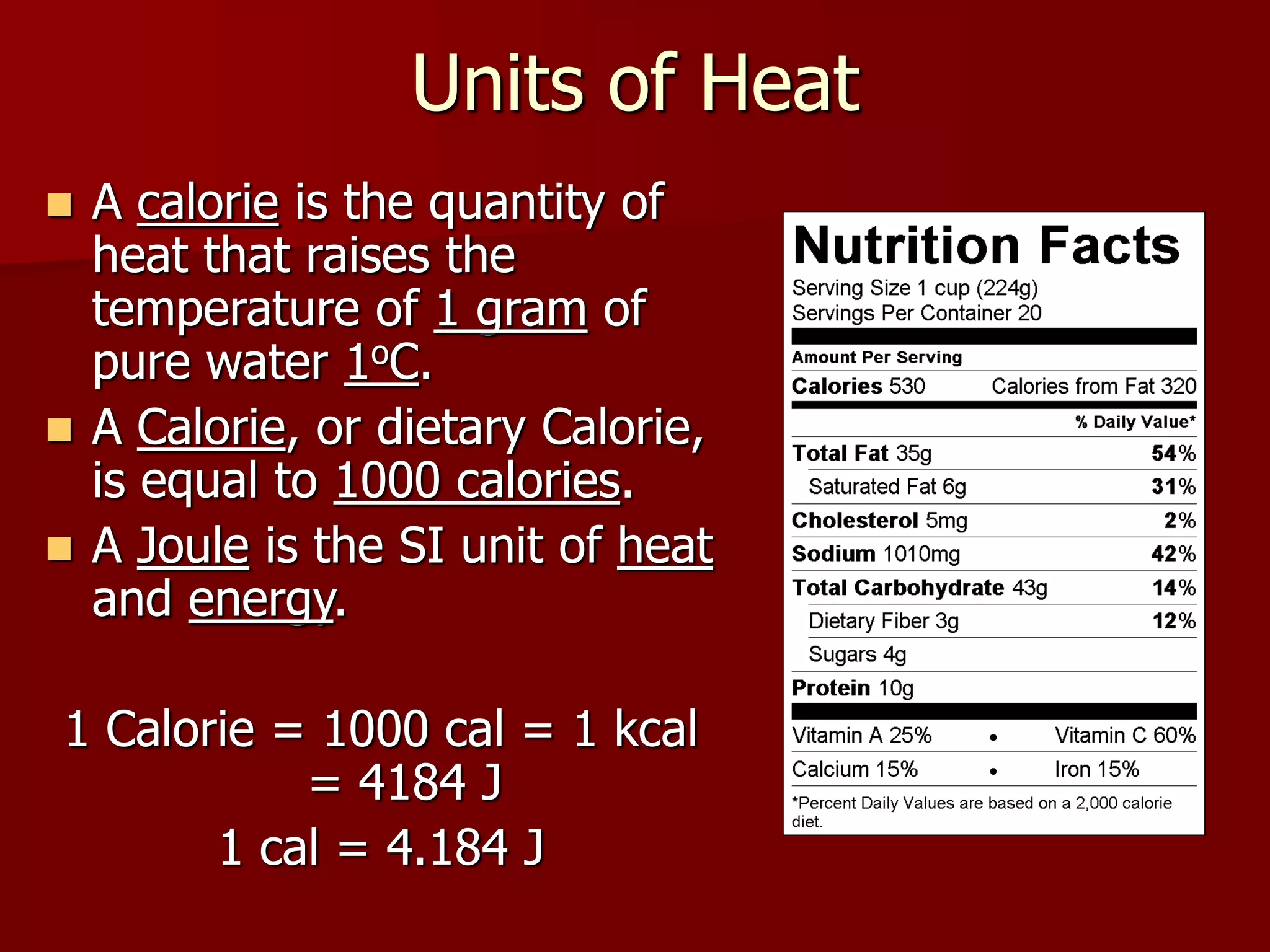
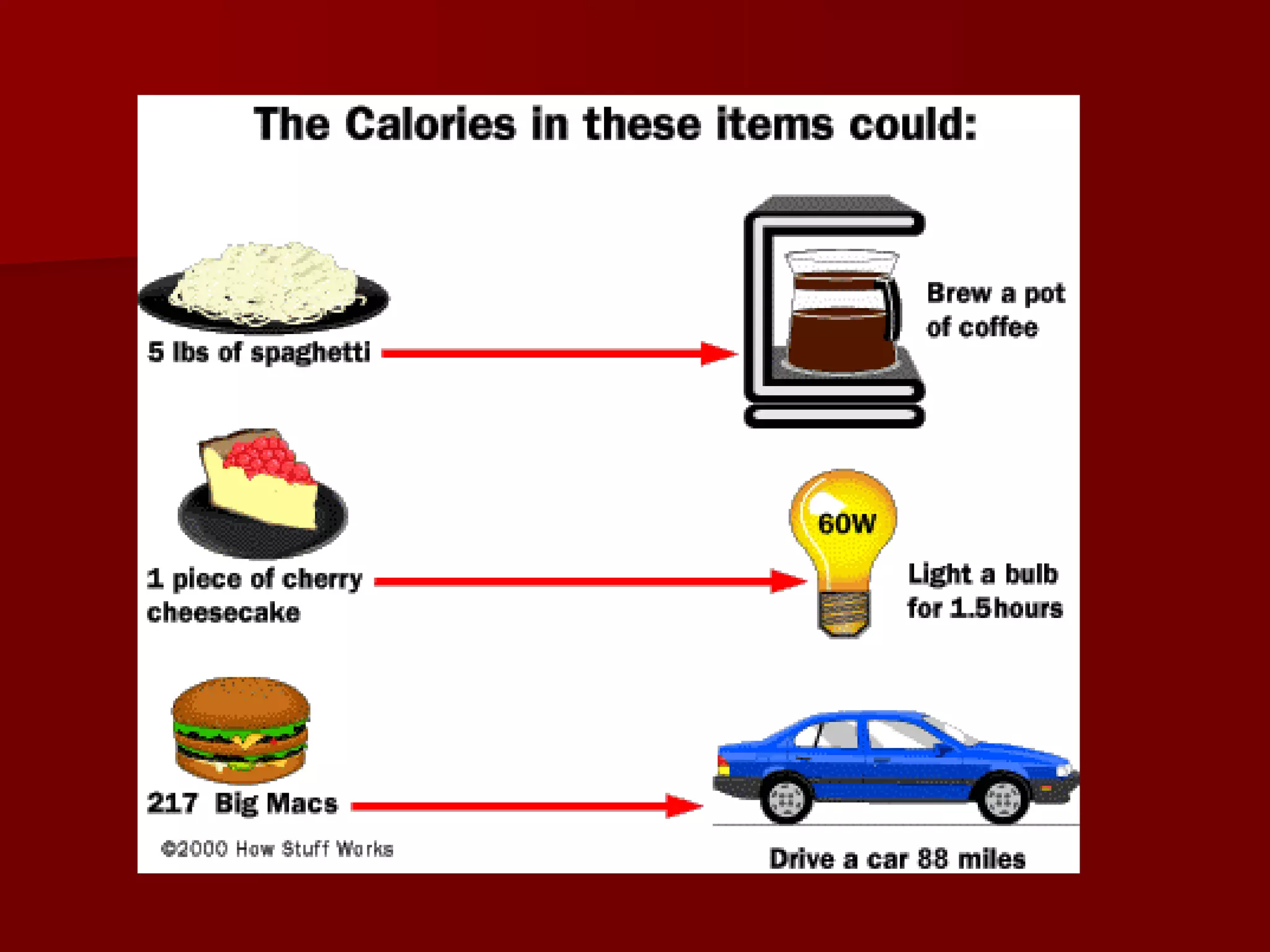
1. Thermochemistry is the study of heat changes that occur during chemical reactions and physical changes of state. It uses concepts such as exothermic and endothermic reactions, enthalpy, and calorimetry.
2. Hess's law states that the heat change of a reaction is equal to the sum of the heat changes of the steps forming that reaction. This allows for calculation of heats of reaction from standard heats of formation.
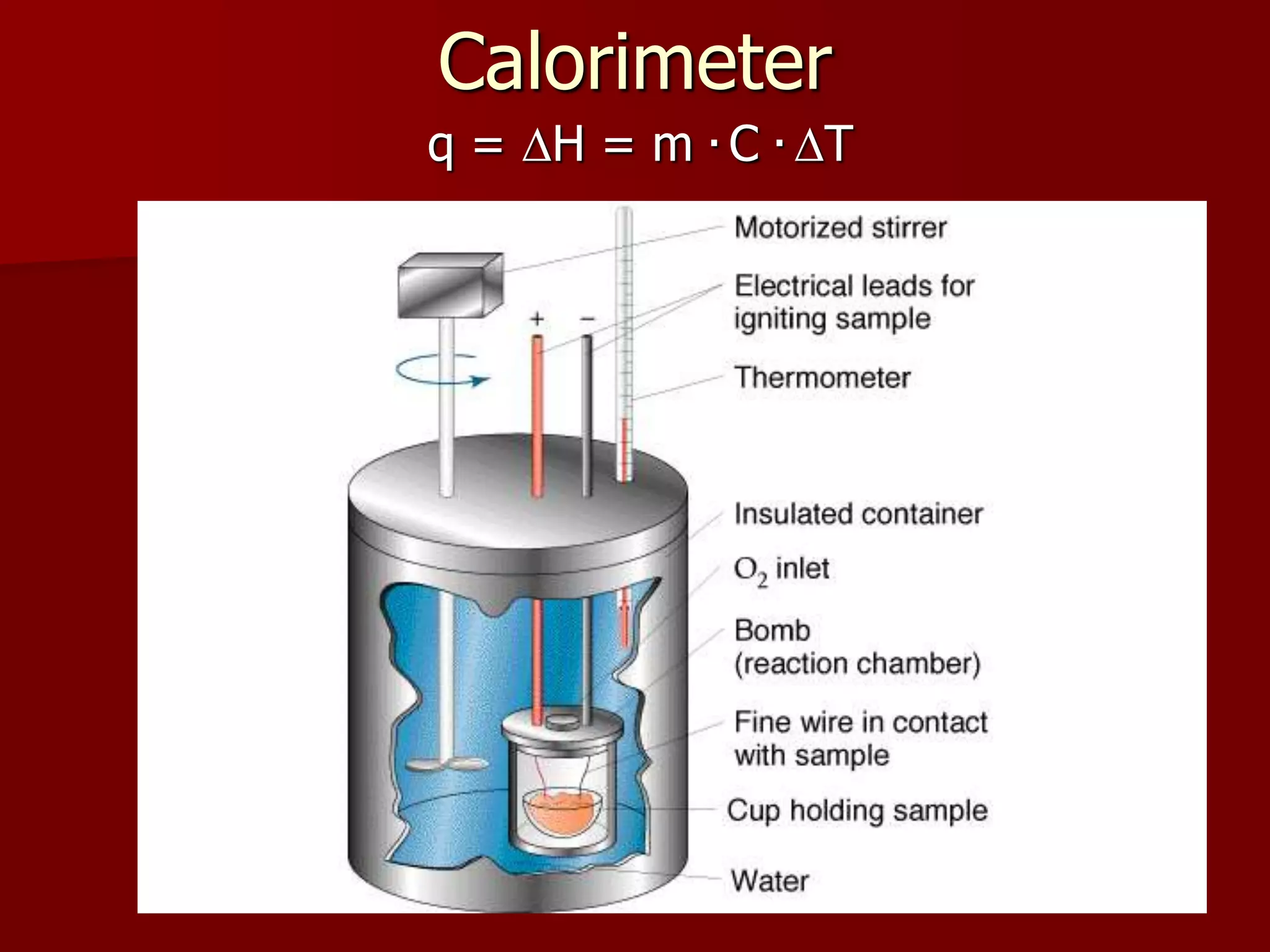
3. Calorimetry is used to directly measure heat changes through the use of calorimeters. Thermochemical equations contain the balanced chemical equation and associated heat change.