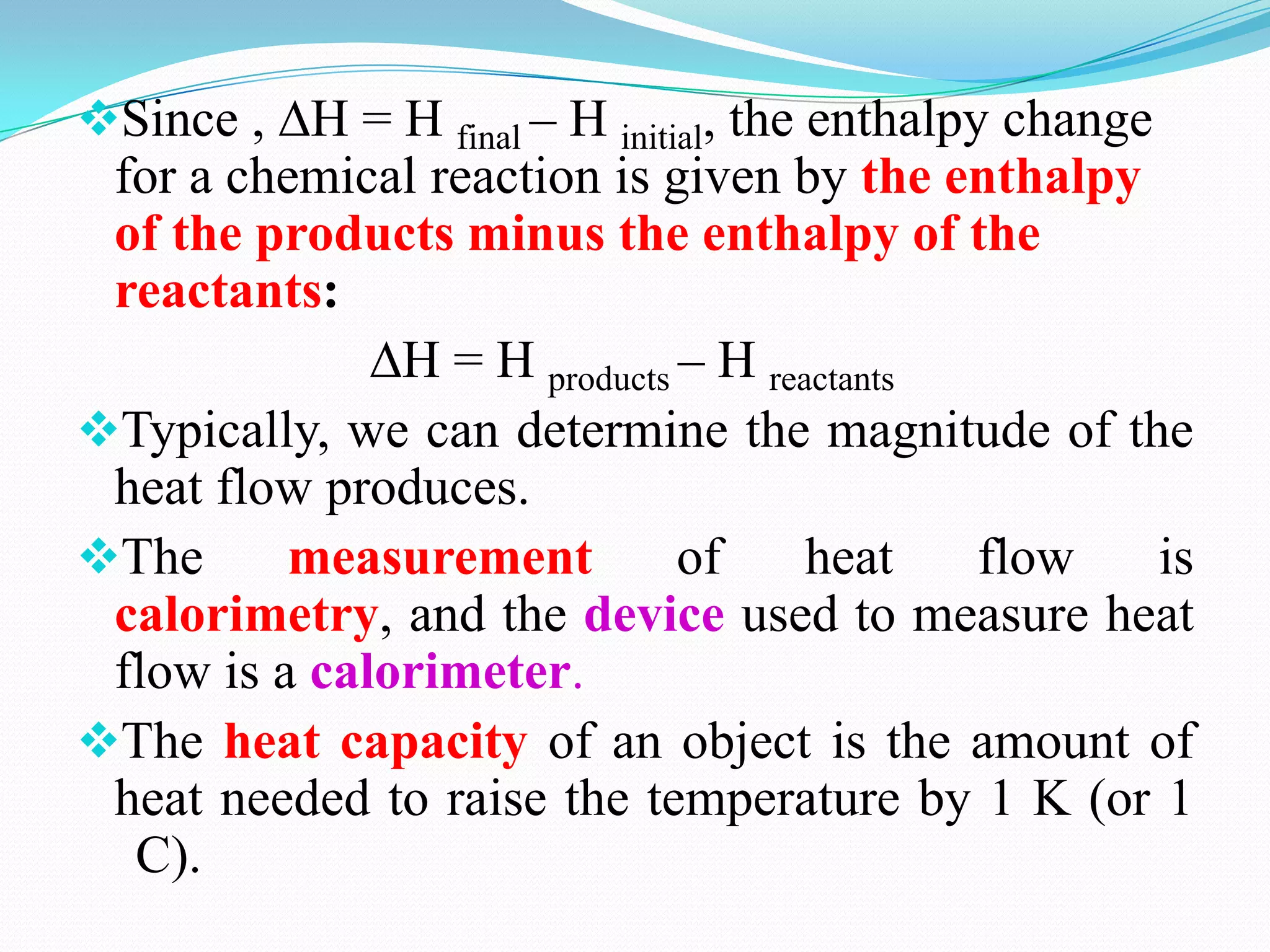
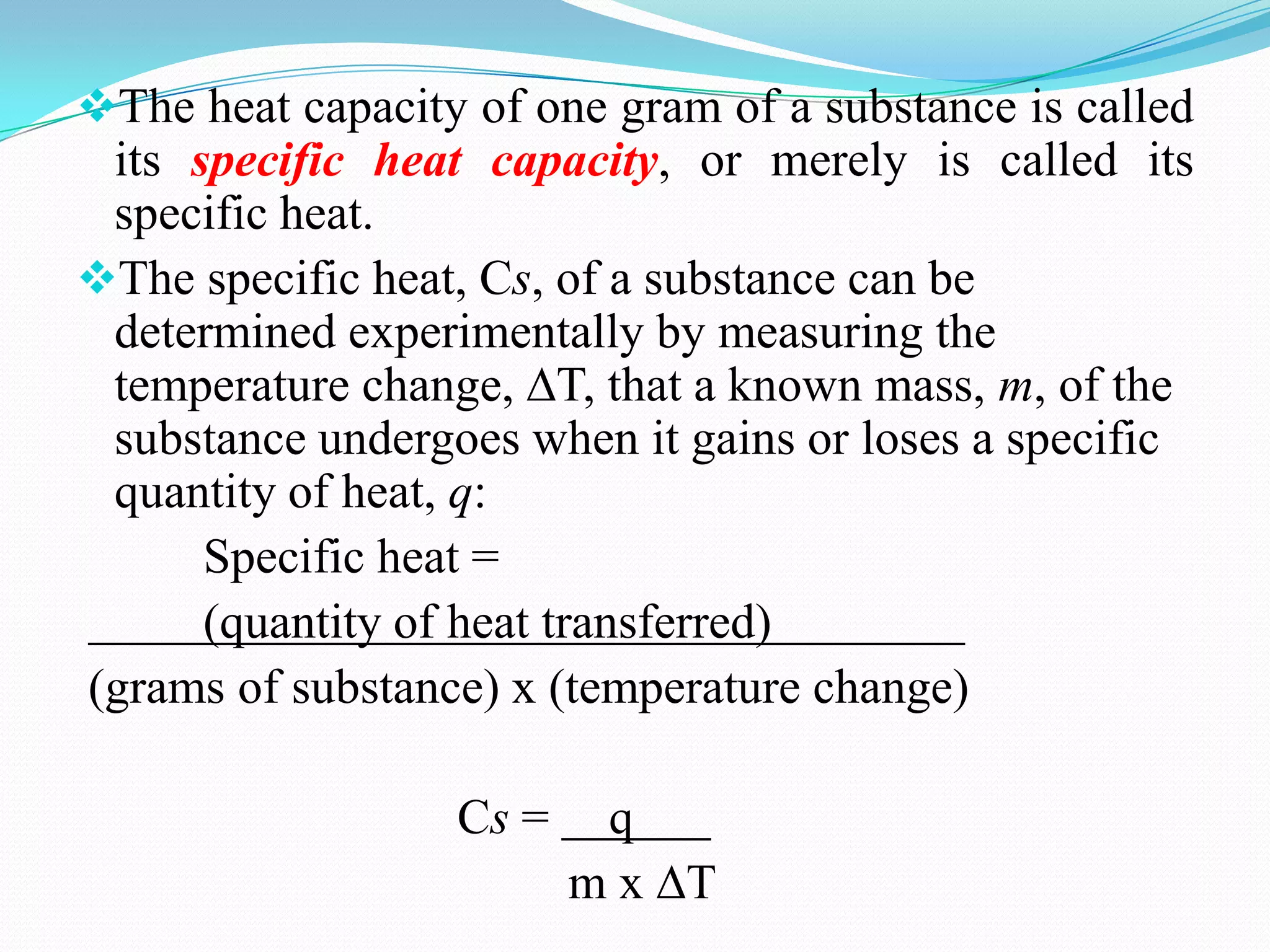
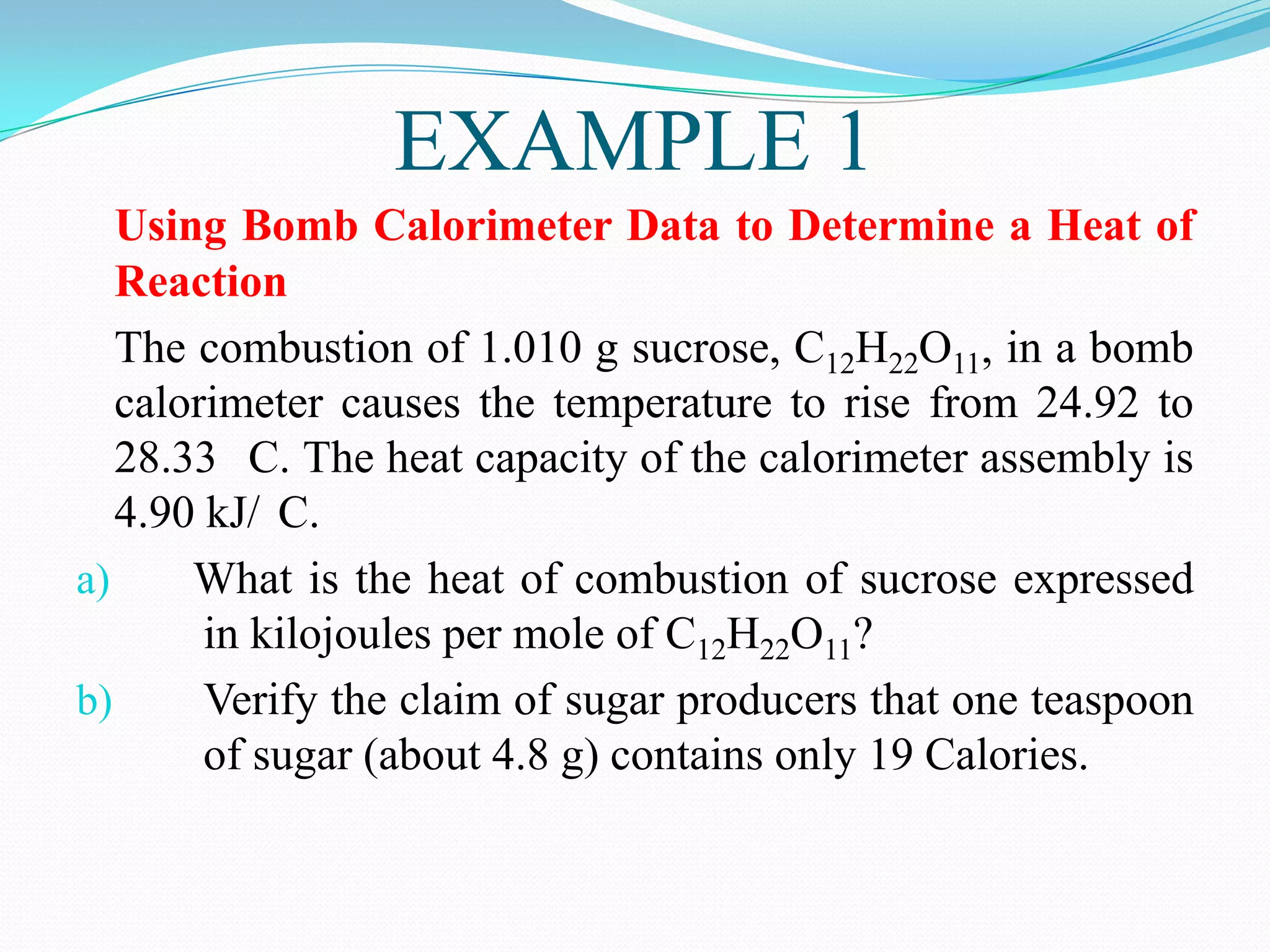
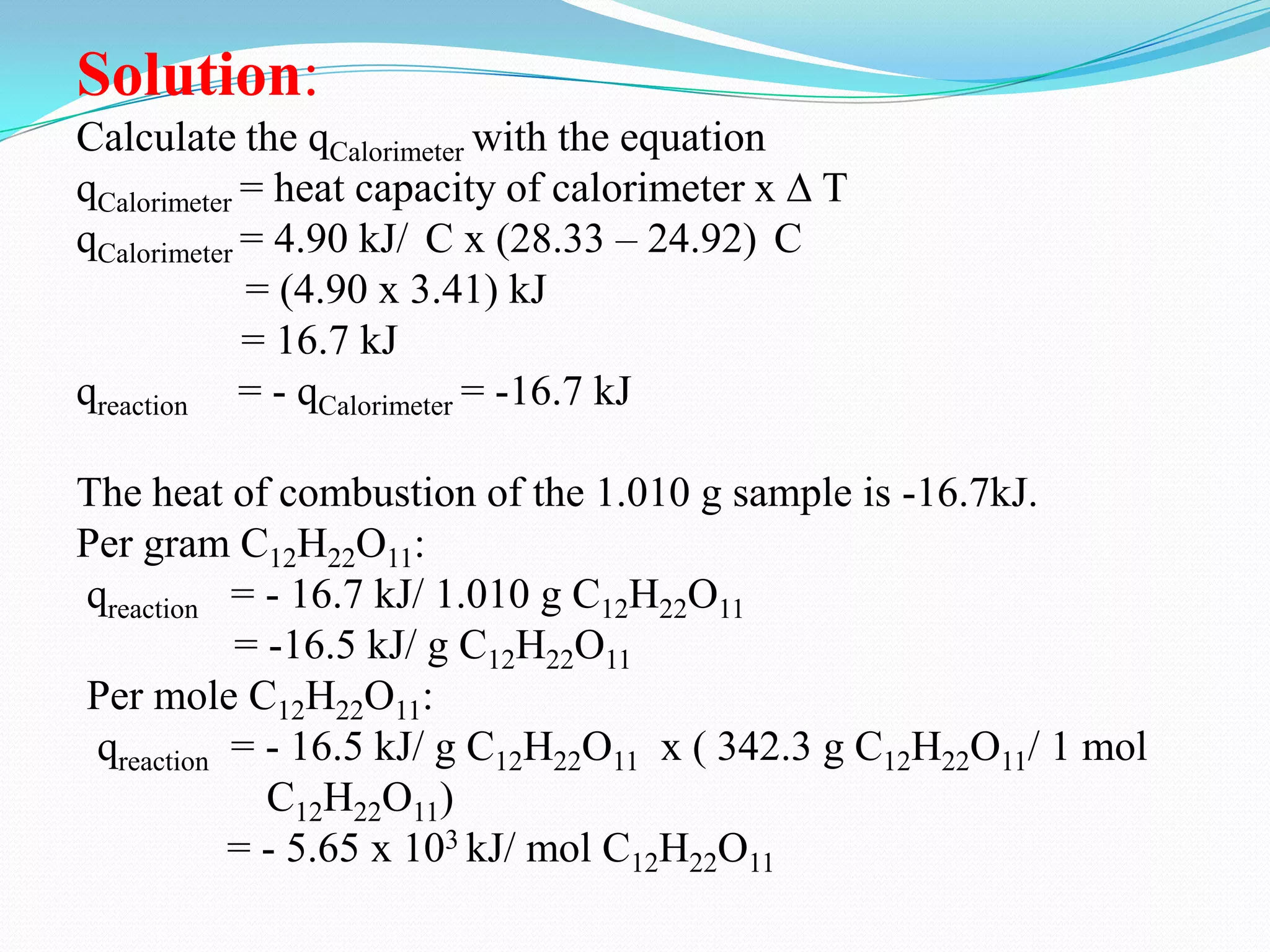
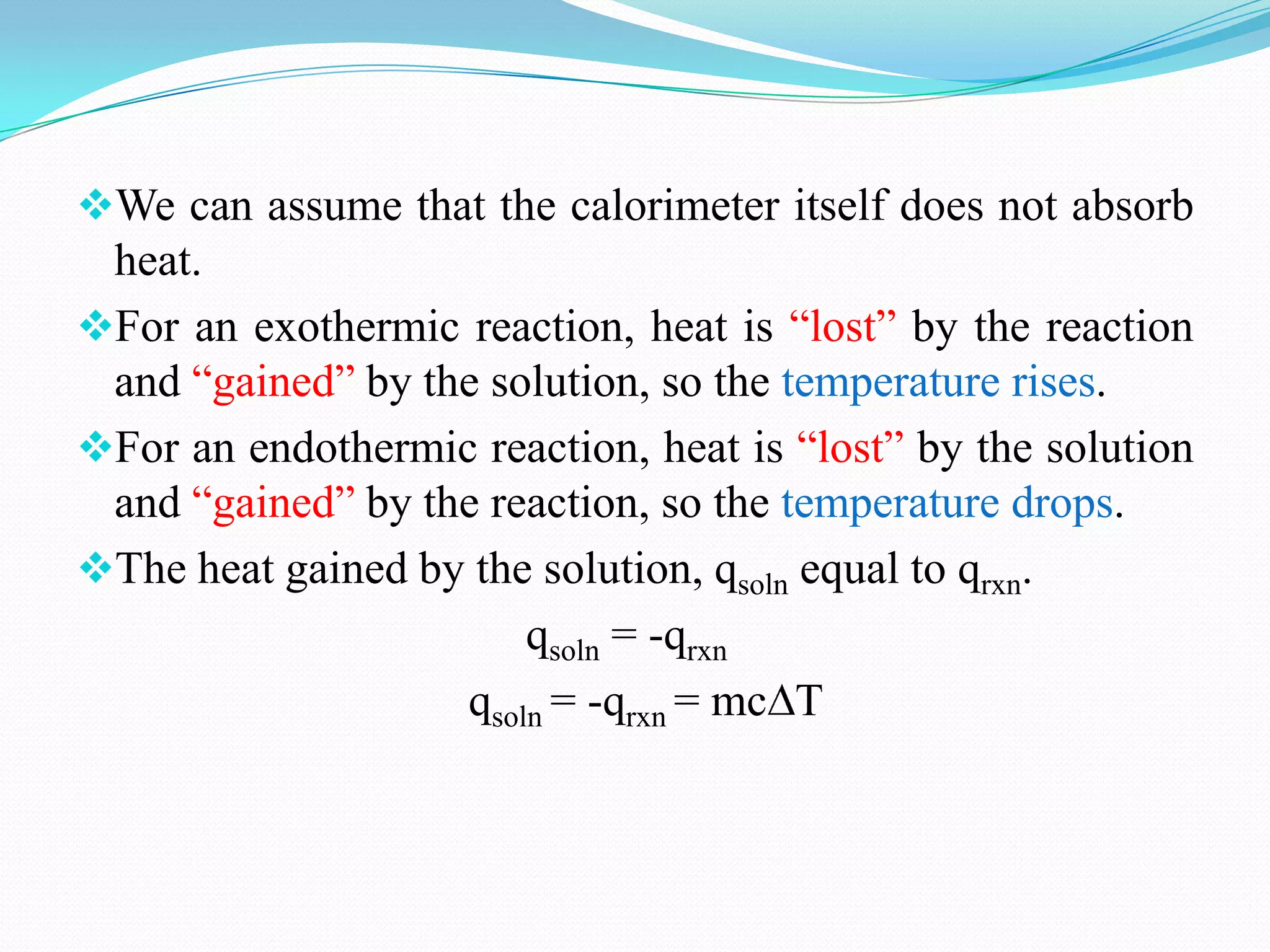
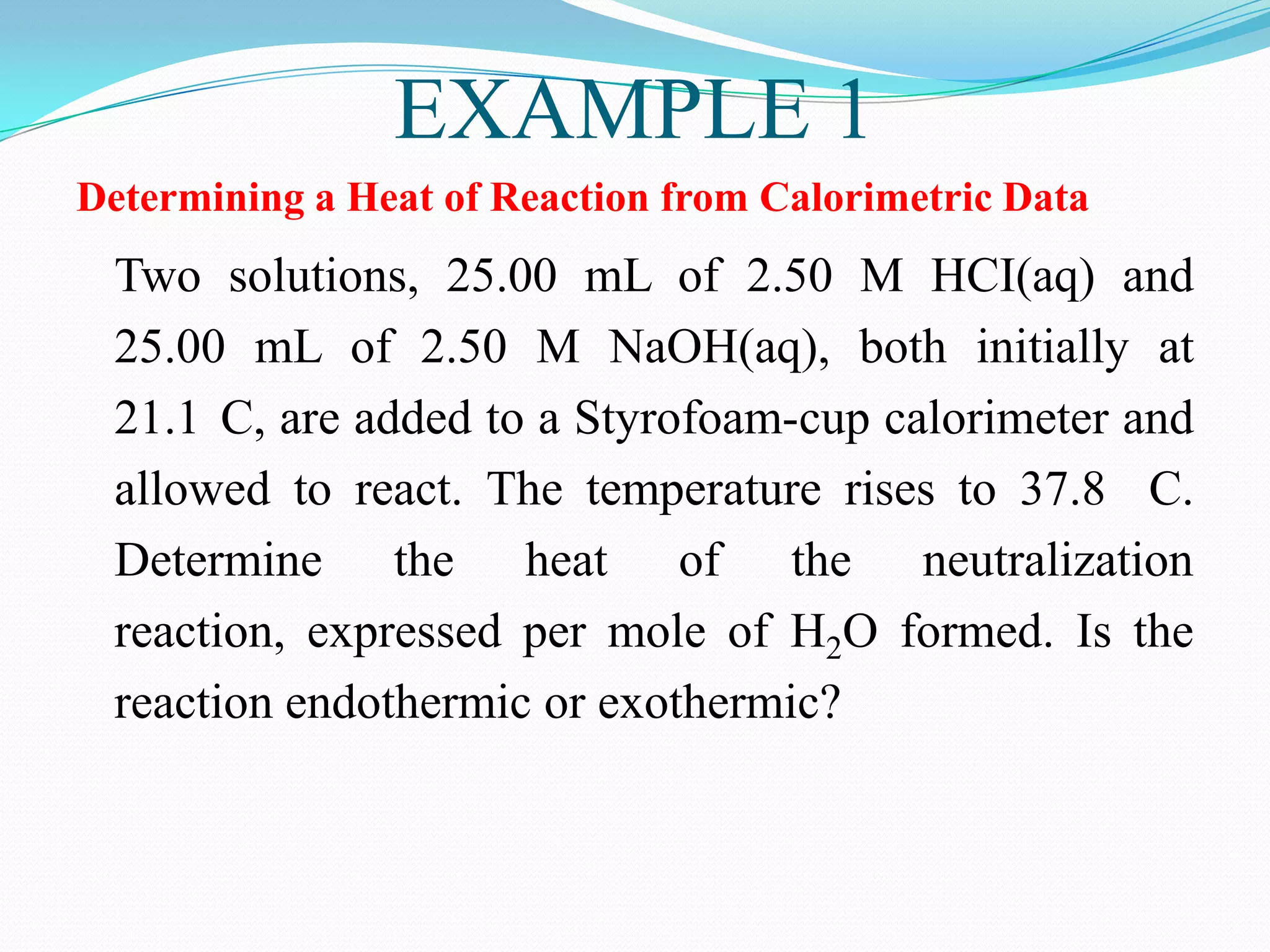
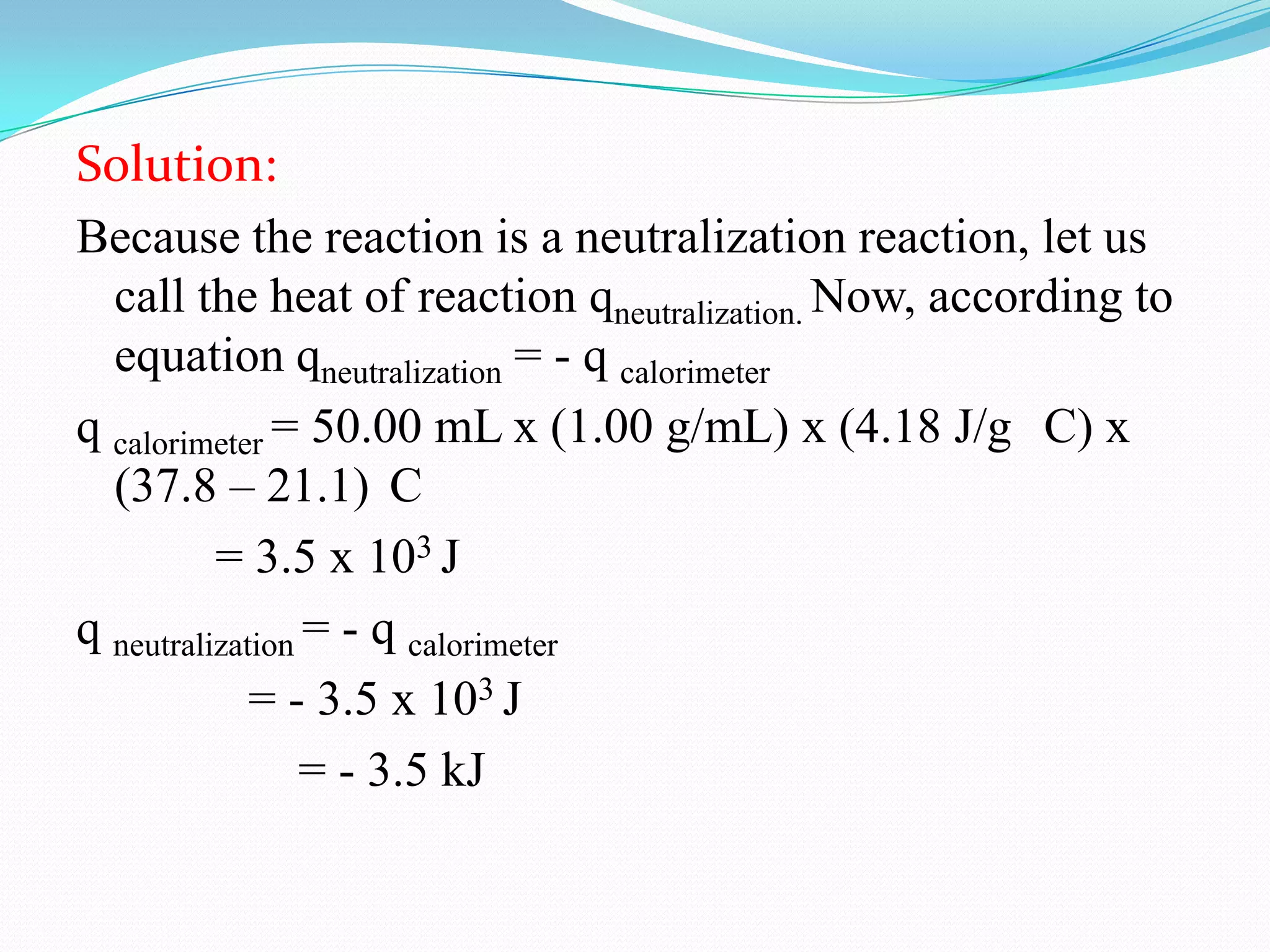
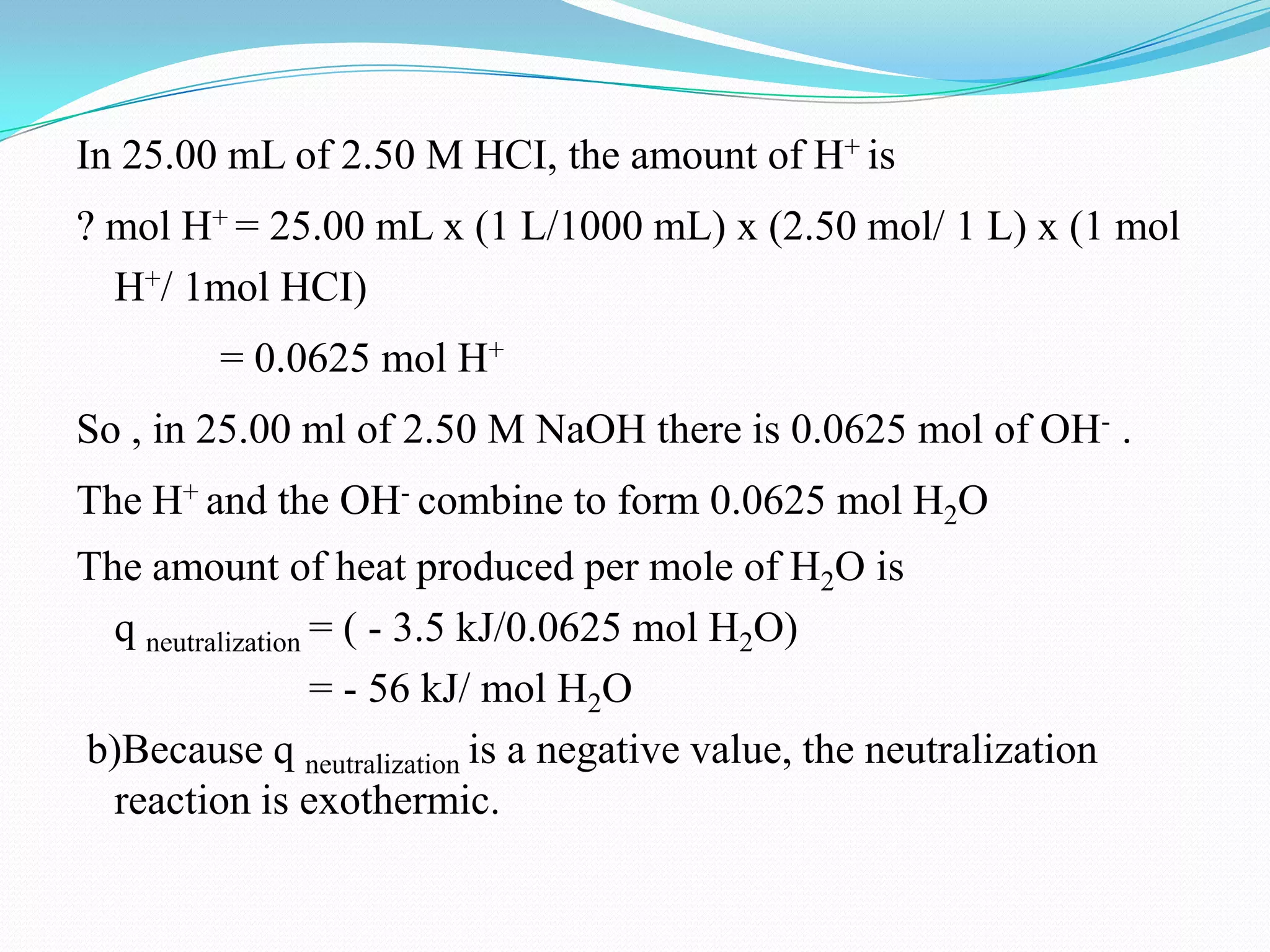
The document describes different types of calorimeters used to measure heat changes in chemical reactions, including bomb calorimeters and coffee cup calorimeters. Bomb calorimeters allow measurement of combustion reactions under constant volume conditions, while coffee cup calorimeters measure heat changes under constant pressure. Examples are provided demonstrating how to use calorimetry data to determine the heat of reaction for a combustion reaction and a neutralization reaction. Both examples show the reactions are exothermic as heat is released.