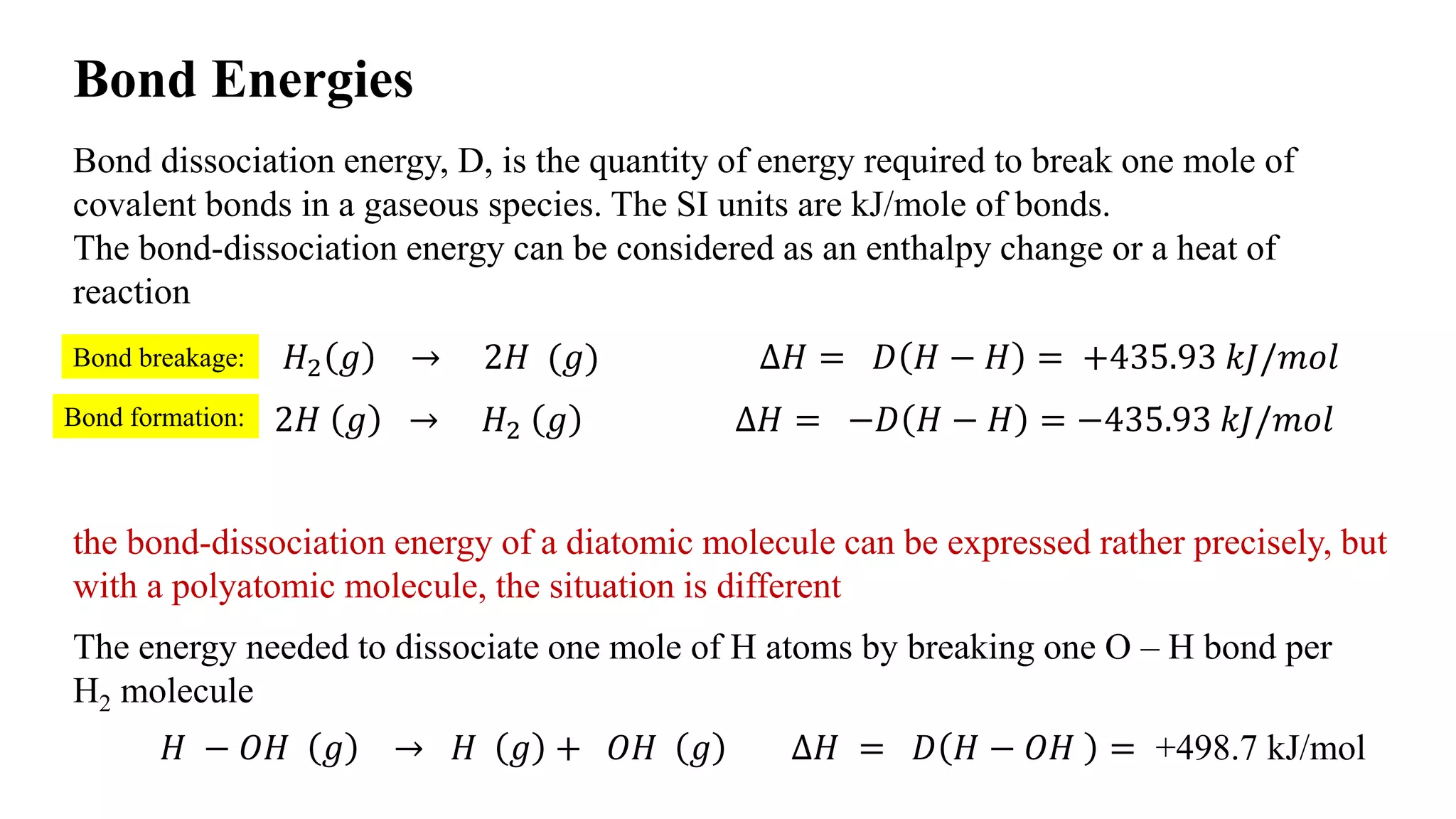
The document discusses bond energies, specifically bond dissociation energies which represent the energy required to break covalent bonds in gaseous species. It details how to calculate bond dissociation energies for diatomic and polyatomic molecules, showing examples including methane and chloromethane reactions. The document also explains how to determine if reactions are exothermic or endothermic based on energy comparisons of bond breakage and formation.

![is different from the energy required to dissociate one mole of H atoms by breaking the
bonds in [OH (g)]:
𝑂 − 𝐻 𝑔 → 𝐻 𝑔 + 𝑂 𝑔 ∆𝐻 = 𝐷 𝑂 − 𝐻 = +428.0 𝑘𝐽/𝑚𝑜𝑙
The two O – H bonds in H2O are identical; therefore, they should have identical
energies.
This energy, which we can call the bond energy in is the average of the two values listed
above: [(498.7 + 428.0)/2 = 463.35]= 463.35 kJ / mol
The bond dissociation energy for O – H bond in CH3OH = 436.8 kJ/mol
An average bond energy is the average of bond-dissociation energies for a number of
different species containing the particular bond. (these values are not precise)](https://image.slidesharecdn.com/bondenergies104-200331205042/75/Bond-energies-10-4-3-2048.jpg)




