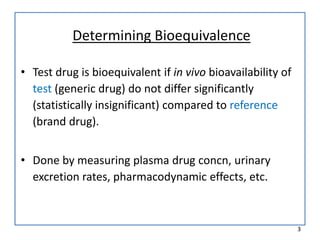
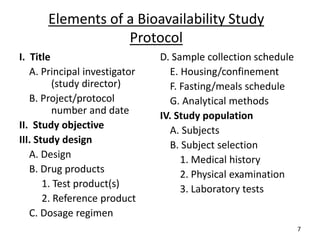
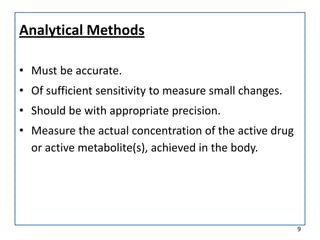
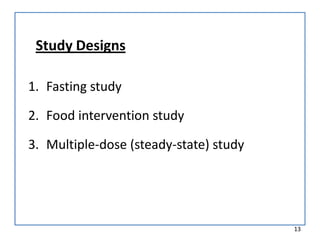
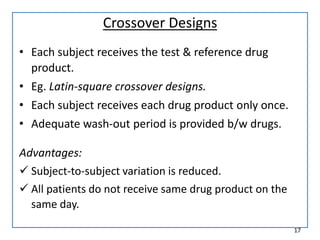
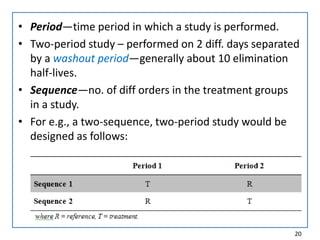
The seminar presented by Muhammed Fahad focused on bioequivalence studies to compare the bioavailability of generic drugs to brand-name products, emphasizing the importance of statistically insignificant differences for therapeutic efficacy. It outlined necessary conditions, study designs, and ethical considerations for establishing bioequivalence, including specific methodologies like fasting, food intervention, and multiple-dose studies. The document also discussed analytical methods, data evaluation techniques, and waivers for in vivo studies under certain conditions.