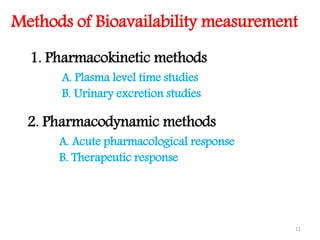
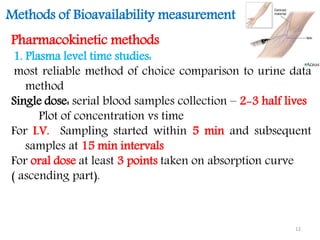
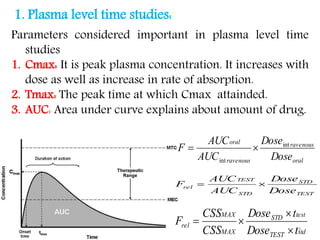
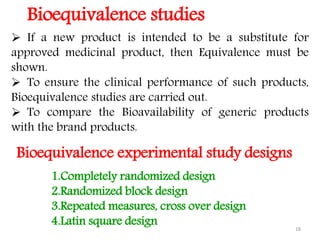
This document discusses bioavailability and bioequivalence concepts including definitions, objectives of bioavailability studies, types of bioavailability studies, and methods of measuring bioavailability. It also covers bioequivalence experimental study designs including completely randomized, randomized block, repeated measures, and Latin square designs. In vitro dissolution studies and developing in vitro-in vivo correlations to help assess bioavailability without human studies are also summarized.