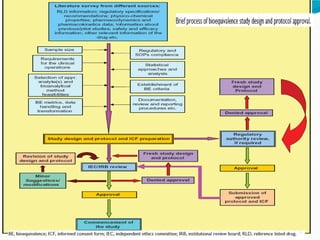
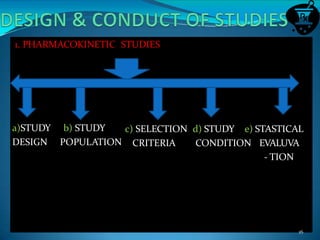
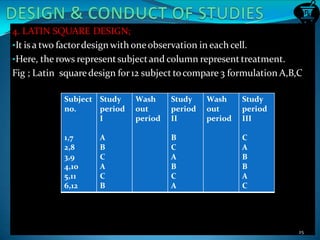
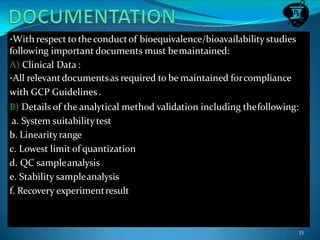
This document discusses guidelines for bioavailability and bioequivalence studies. It defines key terms like bioavailability, bioequivalence, pharmaceutical equivalents and alternatives. It outlines when bioequivalence studies are necessary, such as for modified release drugs, and when they are not required, such as for parenteral solutions. It also describes the different types of studies including pharmacokinetic, pharmacodynamic and clinical endpoint studies. Finally, it provides details on study design, population, conditions and statistical evaluation for pharmacokinetic bioequivalence studies.