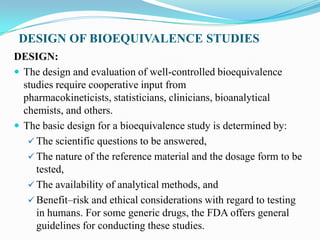
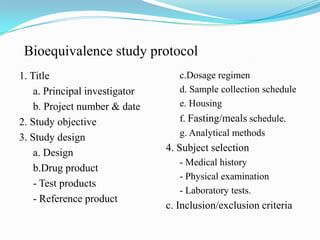
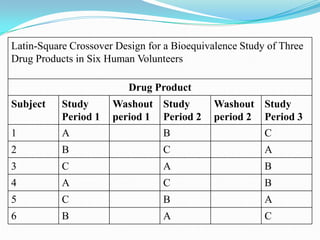
This document provides an introduction to bioequivalence studies, including definitions of key terms, the need for and importance of bioequivalence studies, criteria for establishing a bioequivalence requirement, types of bioequivalence studies, design of bioequivalence studies, evaluation of bioequivalence study results, and clinical significance. It discusses in vivo and in vitro bioequivalence study types and designs, including factors such as single dose, multiple dose, fasting, food effect, and crossover designs. Statistical evaluation methods including ANOVA, confidence intervals, and bioequivalence limits of 80-125% are also summarized.















![Evaluation of bioequivalence studies
Analytical method
• Accuracy, precision and specificity.
• More than one analytical method not be valid.
• Data presented in both tabulated and graphical form
• Plasma drug concentration versus time curve for each drug
product and each subject.
Pharmacokinetic evaluation of the data
• Single dose study:AUC0-t ,AUC0-∞,Tmax and Tmax.
• Multiple dose studies:AUC0-t,Tmax,Cmax,Cmin and percent
fluctuation[100*(Cmax-Cmin)/Cmin].](https://image.slidesharecdn.com/bioequivalence-112070804009-130119105915-phpapp01/85/Bioequivalence-112070804009-25-320.jpg)