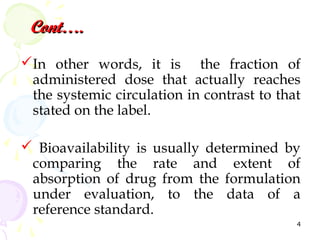
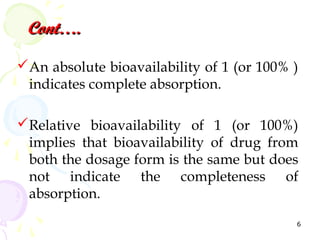
Bioavailability is defined as the fraction of an administered drug dose that reaches systemic circulation. It is determined by comparing the rate and extent of absorption of a test formulation to a reference standard, usually an intravenous dose. Key considerations in bioavailability studies include selecting appropriate subjects, study design such as single vs multiple dose studies and washout periods, and ensuring the analytical method can detect drug and metabolite levels. The goal is to understand how formulation characteristics impact the biological performance of the drug.