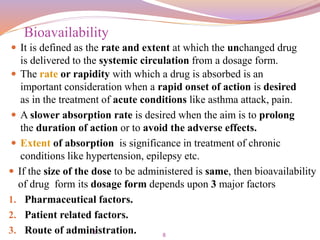
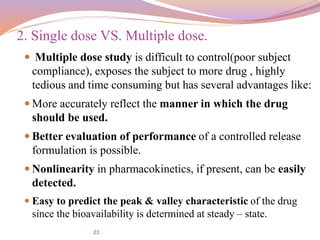
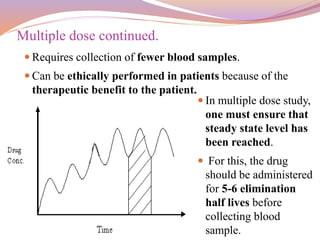
This document provides information about bioavailability and bioequivalence studies. It defines bioavailability as the rate and extent to which a drug enters systemic circulation from a dosage form. Factors influencing bioavailability include pharmaceutical, patient, and route of administration factors. The objectives of bioavailability studies are discussed, including determining the influence of excipients and possible drug interactions. Types of bioavailability study designs covered include absolute vs relative bioavailability, single vs multiple dose studies, and using healthy subjects vs patients. Methods for measuring bioavailability through pharmacokinetic studies of plasma drug levels and urinary excretion studies are also summarized.







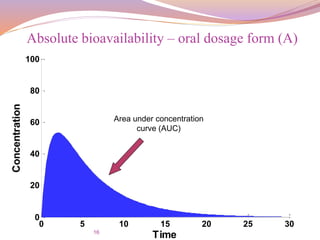
![17
0 5 10 15 20 25 30
0
20
40
60
80
100
Time
Concentration
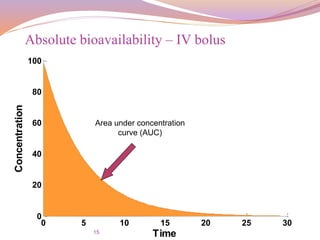
For the same dose(IV vs. Oral),
the bioavailability is given by:
Absolute bioavailability of IV and Oral dosage form
F =
𝐴𝑈𝐶 𝑜𝑟𝑎𝑙
∗
𝑑𝑜𝑠𝑒 𝐼
.
𝑉
𝐴𝑈𝐶 𝐼
.
𝑉
∗ [𝑑𝑜𝑠𝑒] 𝑜𝑟𝑎𝑙](https://image.slidesharecdn.com/baandbe-180413200712/85/Bioavailability-and-Bioequivalence-17-320.jpg)

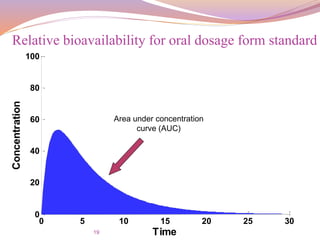
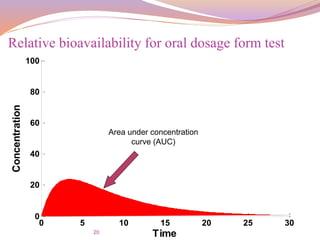
![21
0 5 10 15 20 25 30
0
20
40
60
80
100
Time
Concentration
For the same dose (Oral vs. Oral)
The bioavailability is given by:
F =
𝐴𝑈𝐶 𝑇𝑒𝑠𝑡
∗
𝑑𝑜𝑠𝑒 𝑆𝑡𝑑
𝐴𝑈𝐶 𝑆𝑡𝑑
∗ [
𝑑𝑜𝑠𝑒] 𝑇𝑒𝑠𝑡
Relative bioavailability for oral dosage form standard
and test](https://image.slidesharecdn.com/baandbe-180413200712/85/Bioavailability-and-Bioequivalence-21-320.jpg)
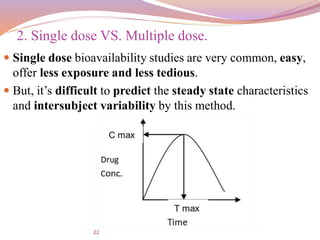



![30
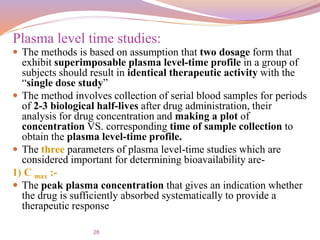
T max:-
The peak time that gives an indication of the rate of
absorption.
It decreases as the rate of absorption increase.
AUC:-
The area under the plasma level time curve that gives a
measure of the extent of absorption or the amount of drug
that reaches the systemic circulation.
The extent of bioavailability can be determined by the
following equations
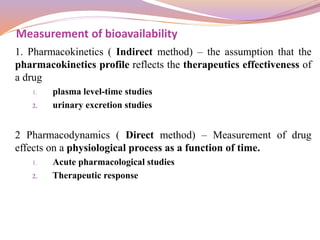
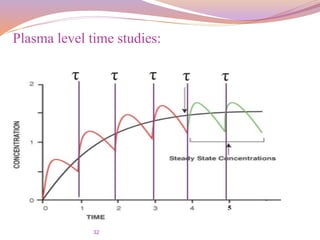
Plasma level time studies:
F =
𝐴𝑈𝐶 𝑜𝑟𝑎𝑙 ∗
𝑑𝑜𝑠𝑒 𝐼. 𝑉
𝐴𝑈𝐶 𝐼. 𝑉
∗ [𝑑𝑜𝑠𝑒] 𝑜𝑟𝑎𝑙
F =
𝐴𝑈𝐶 𝑇𝑒𝑠𝑡 ∗
𝑑𝑜𝑠𝑒 𝑆𝑡𝑑
𝐴𝑈𝐶 𝑆𝑡𝑑 ∗ [
𝑑𝑜𝑠𝑒] 𝑇𝑒𝑠𝑡](https://image.slidesharecdn.com/baandbe-180413200712/85/Bioavailability-and-Bioequivalence-30-320.jpg)

![35
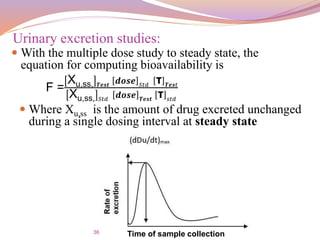
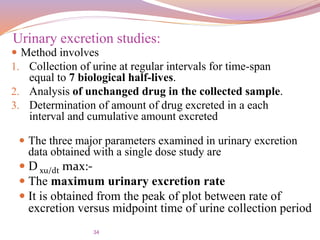
Urinary excretion studies:
Tu max:-
The time for maximum excretion rate
It is analogous to the T max of the plasma level data
Its value decreases as the absorption rate increases
The extent of bioavailability can be determined by the
following equations
X∞
u:-
The cumulative amount of drug excreted in the urine
It is related to the AUC of plasma level data and increases as
the extent of absorption increases
F =
X∞
u 𝑜𝑟𝑎𝑙 ∗
𝑑𝑜𝑠𝑒 𝐼. 𝑉
X∞
u 𝐼
.
𝑉 ∗ [𝑑𝑜𝑠𝑒] 𝑜𝑟𝑎𝑙
F =
X∞
u 𝑇𝑒𝑠𝑡 ∗
𝑑𝑜𝑠𝑒 𝑆𝑡𝑑
X∞
u 𝑆𝑡𝑑
∗ [
𝑑𝑜𝑠𝑒] 𝑇𝑒𝑠𝑡](https://image.slidesharecdn.com/baandbe-180413200712/85/Bioavailability-and-Bioequivalence-35-320.jpg)