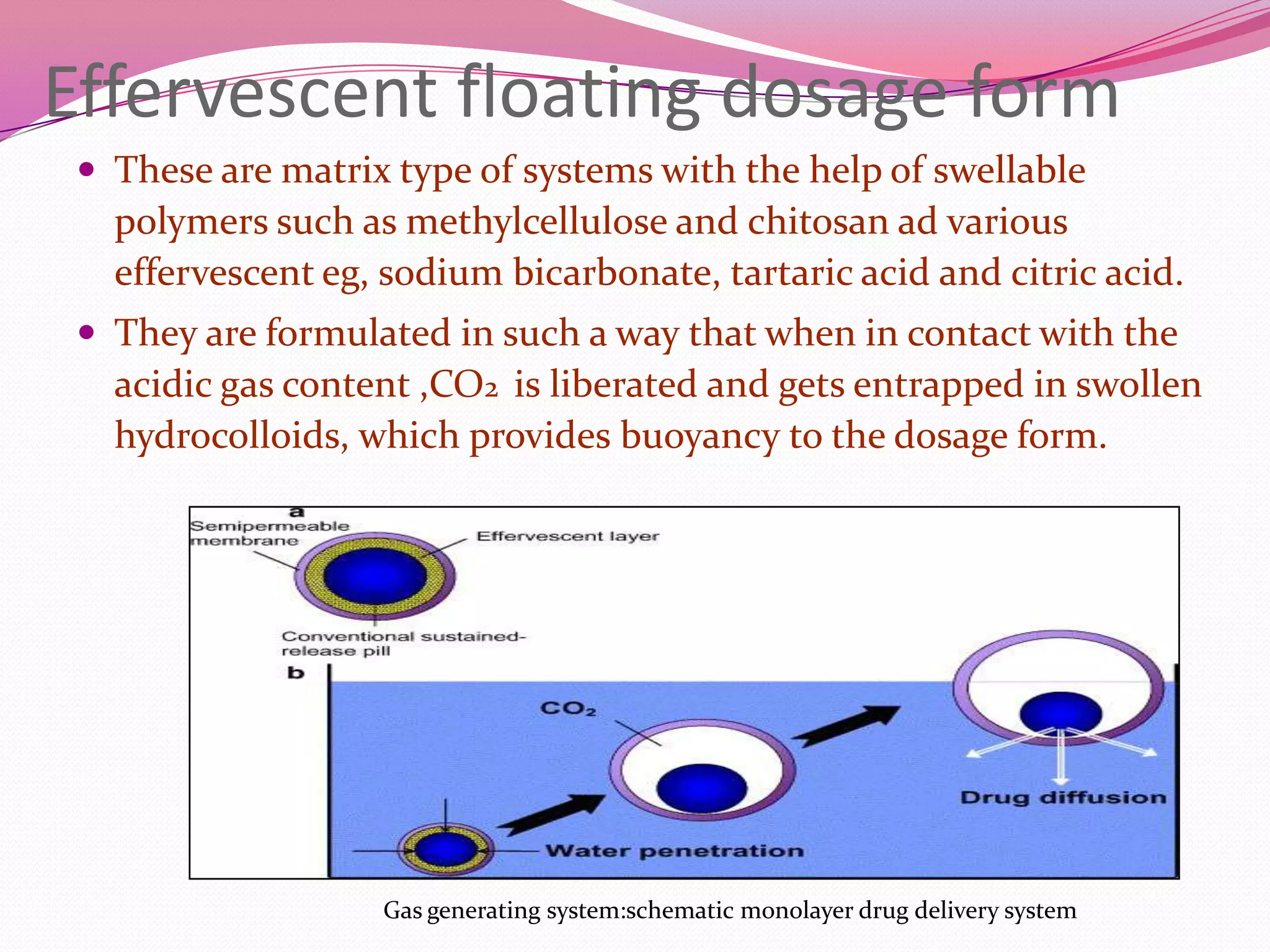
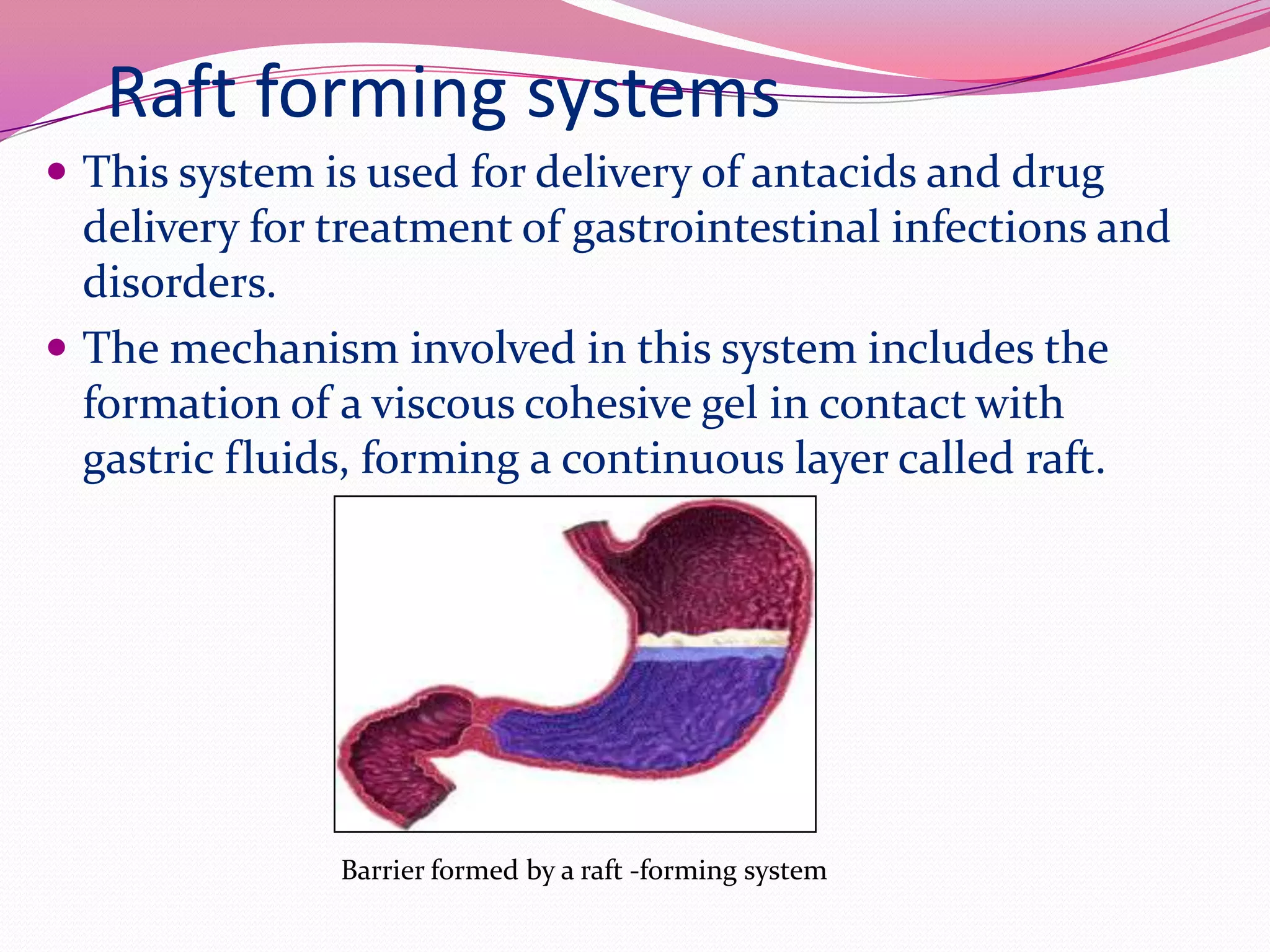
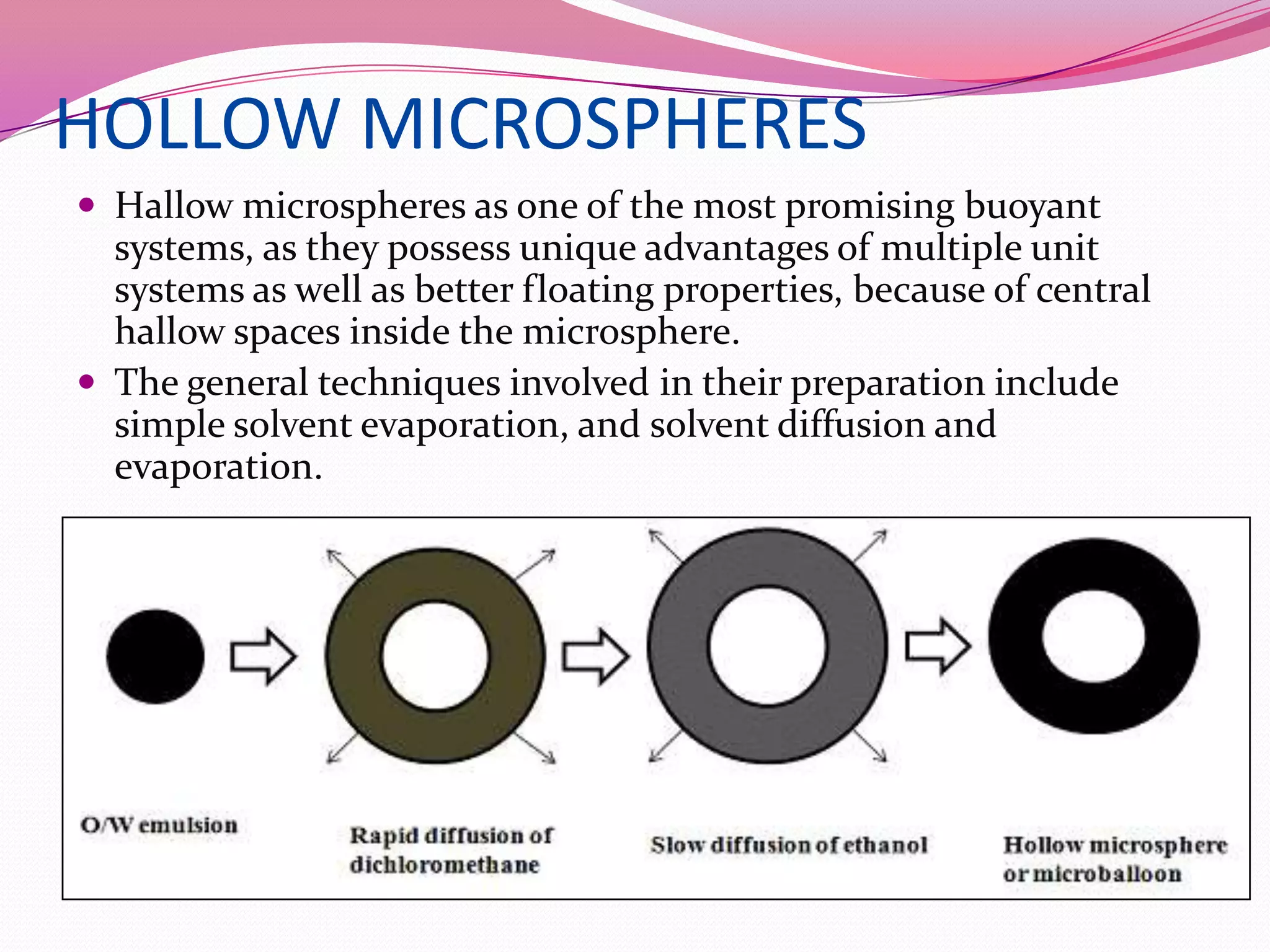
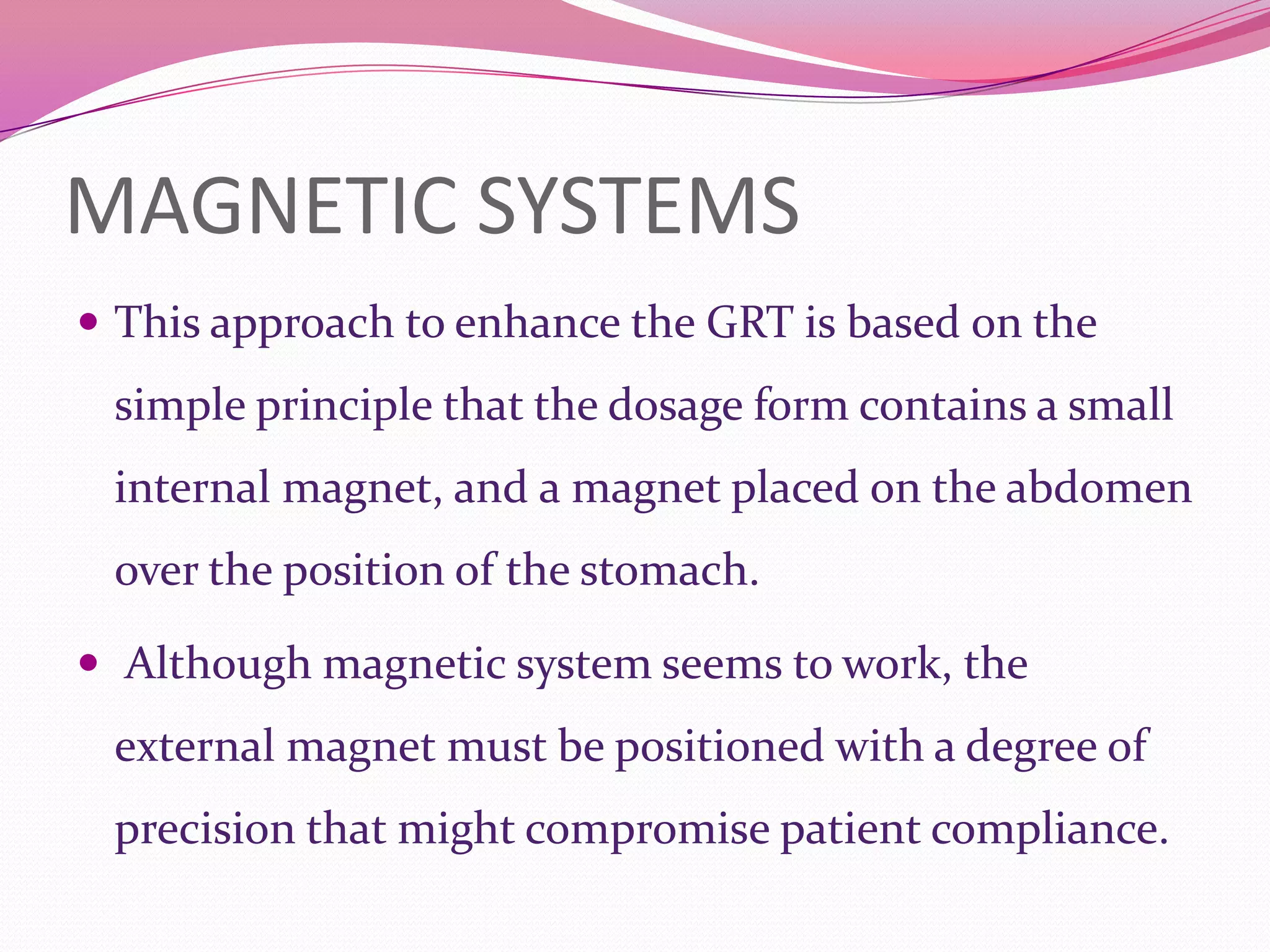
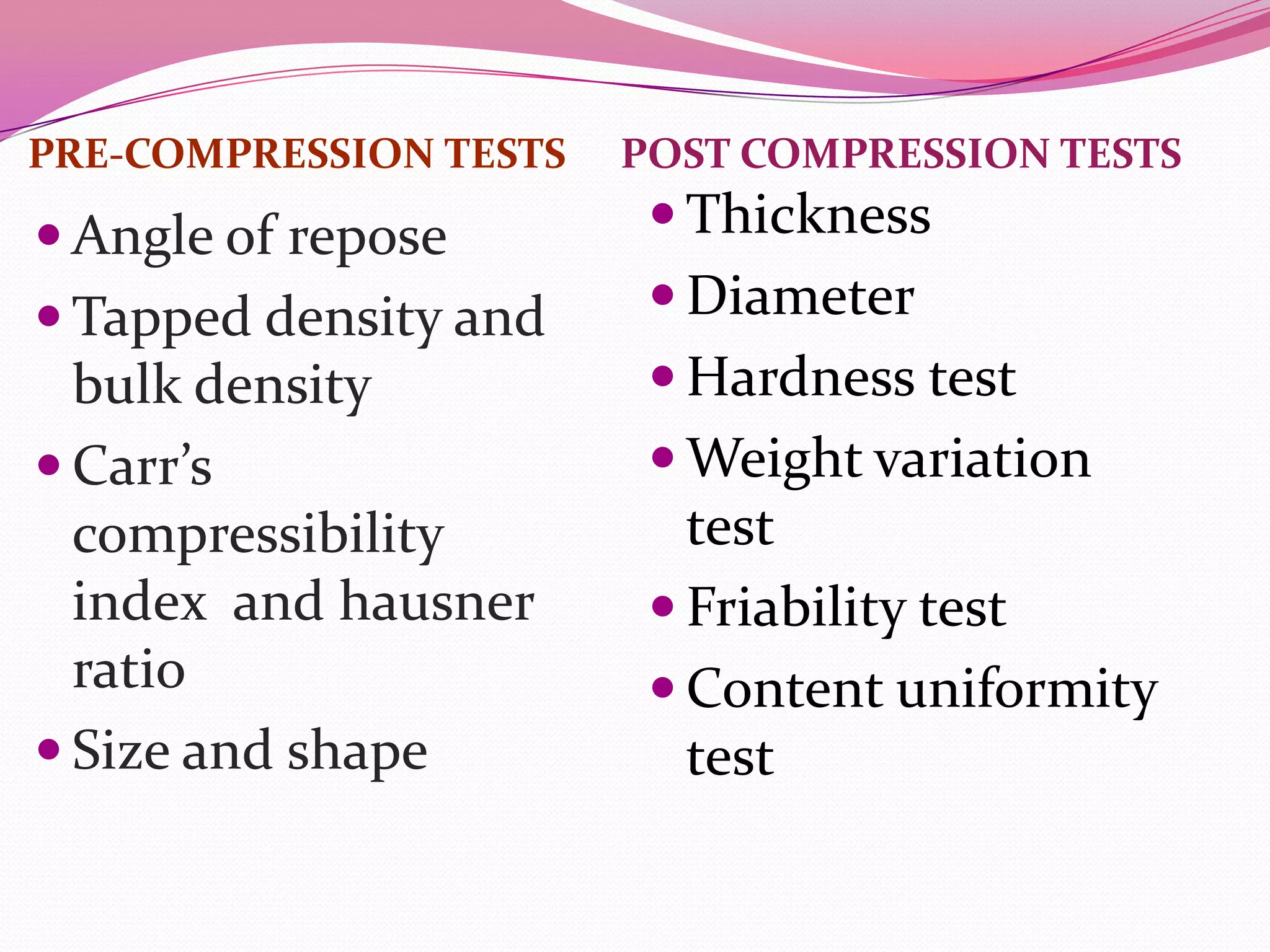
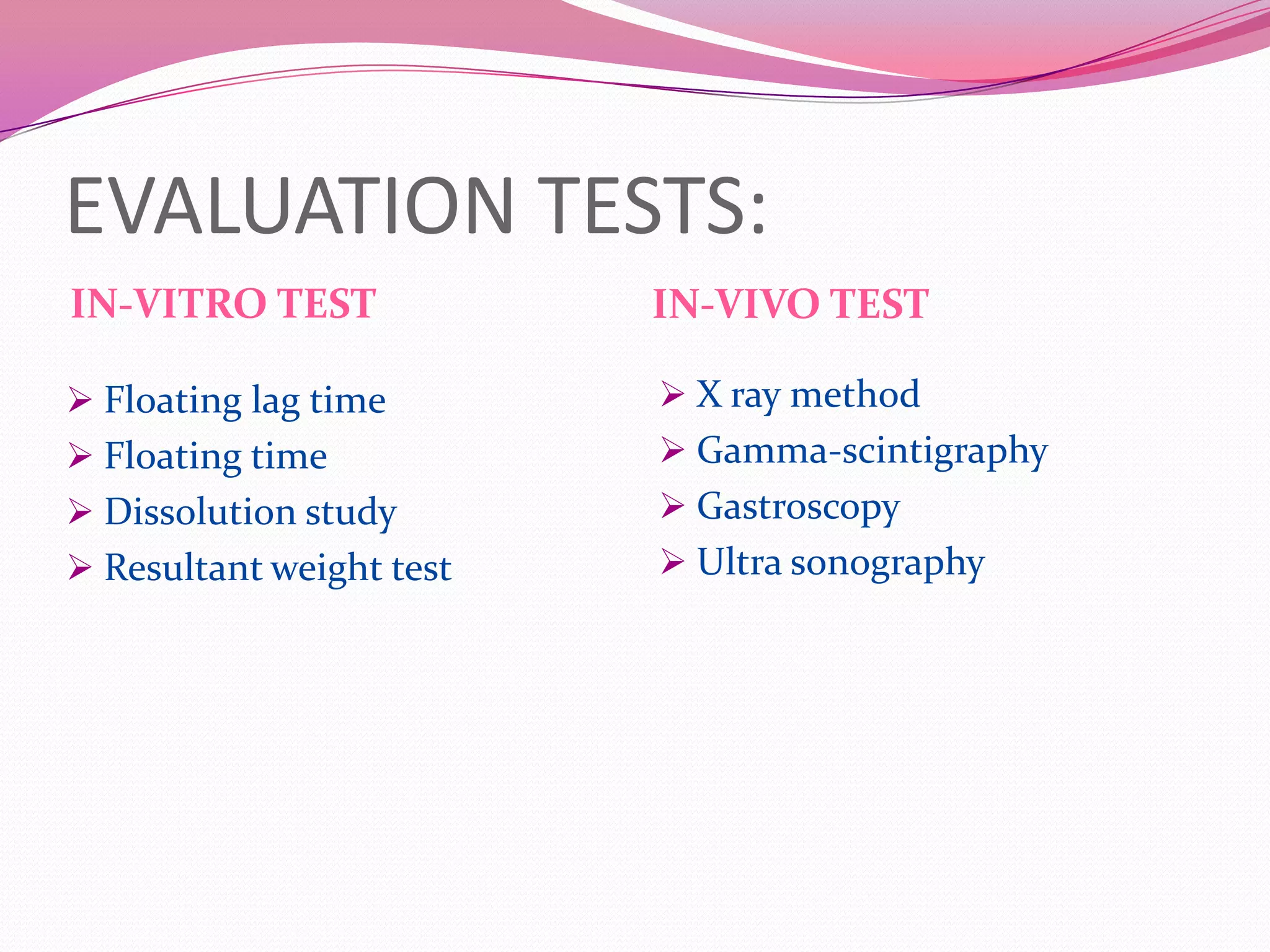
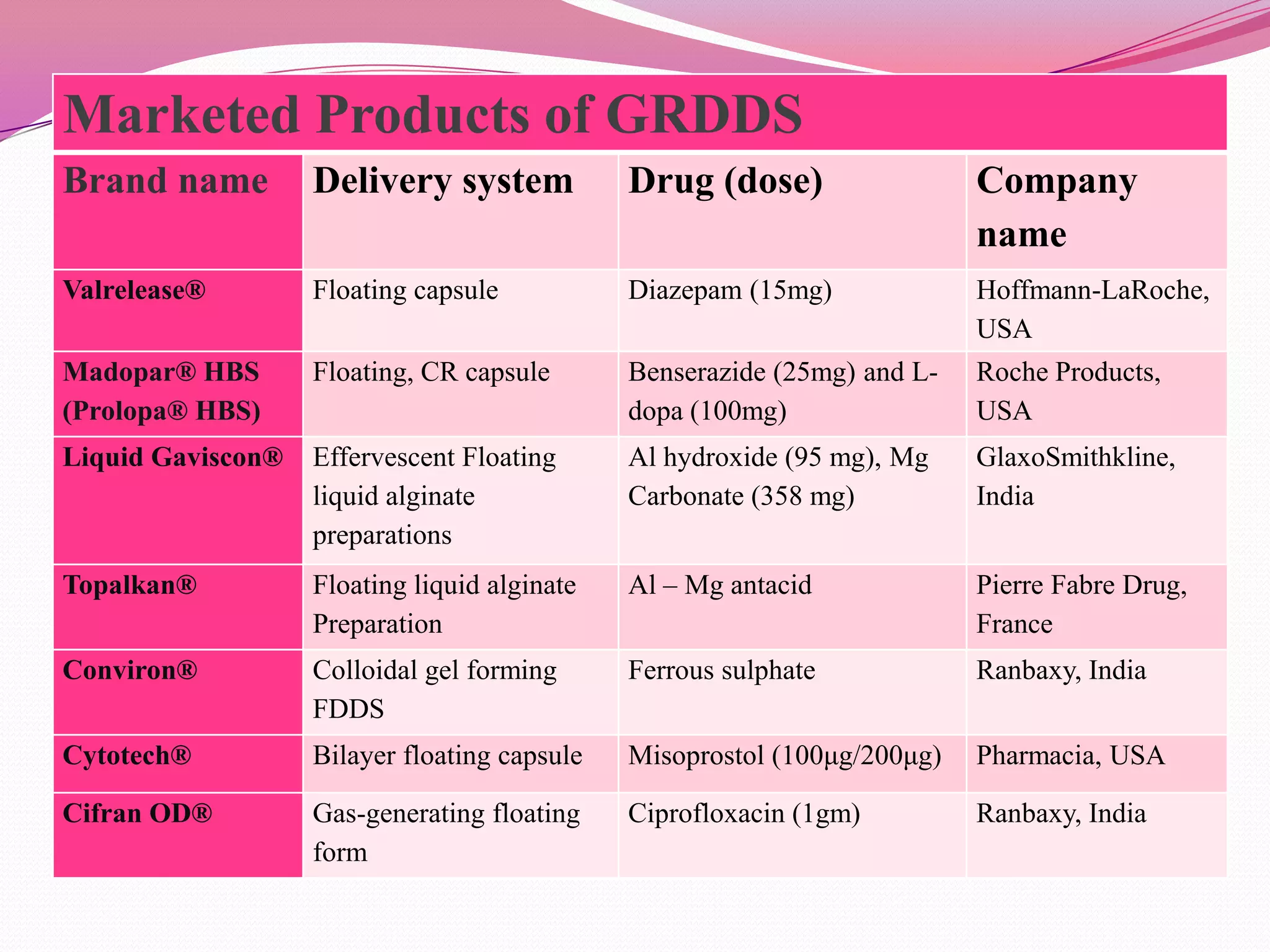
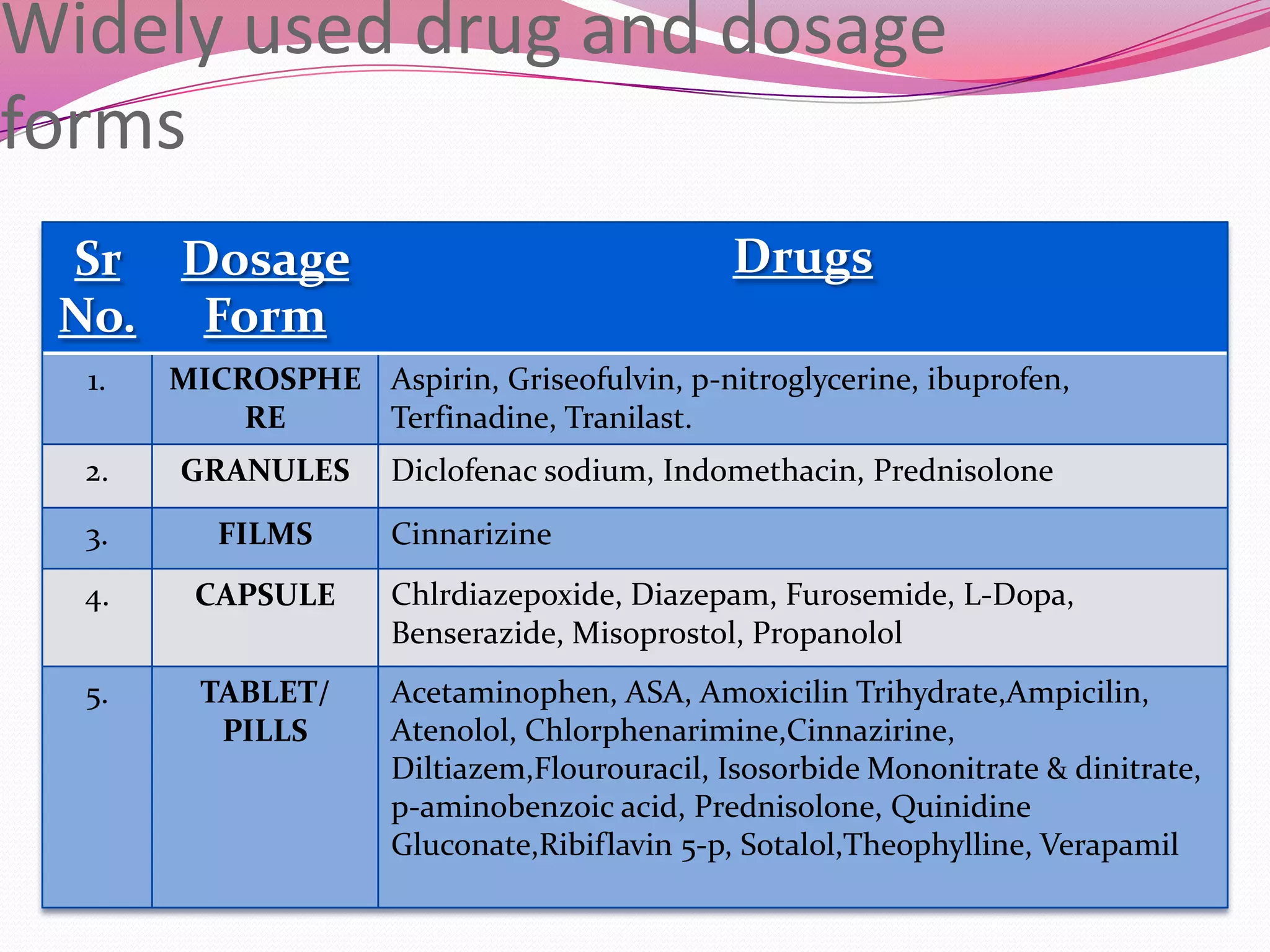
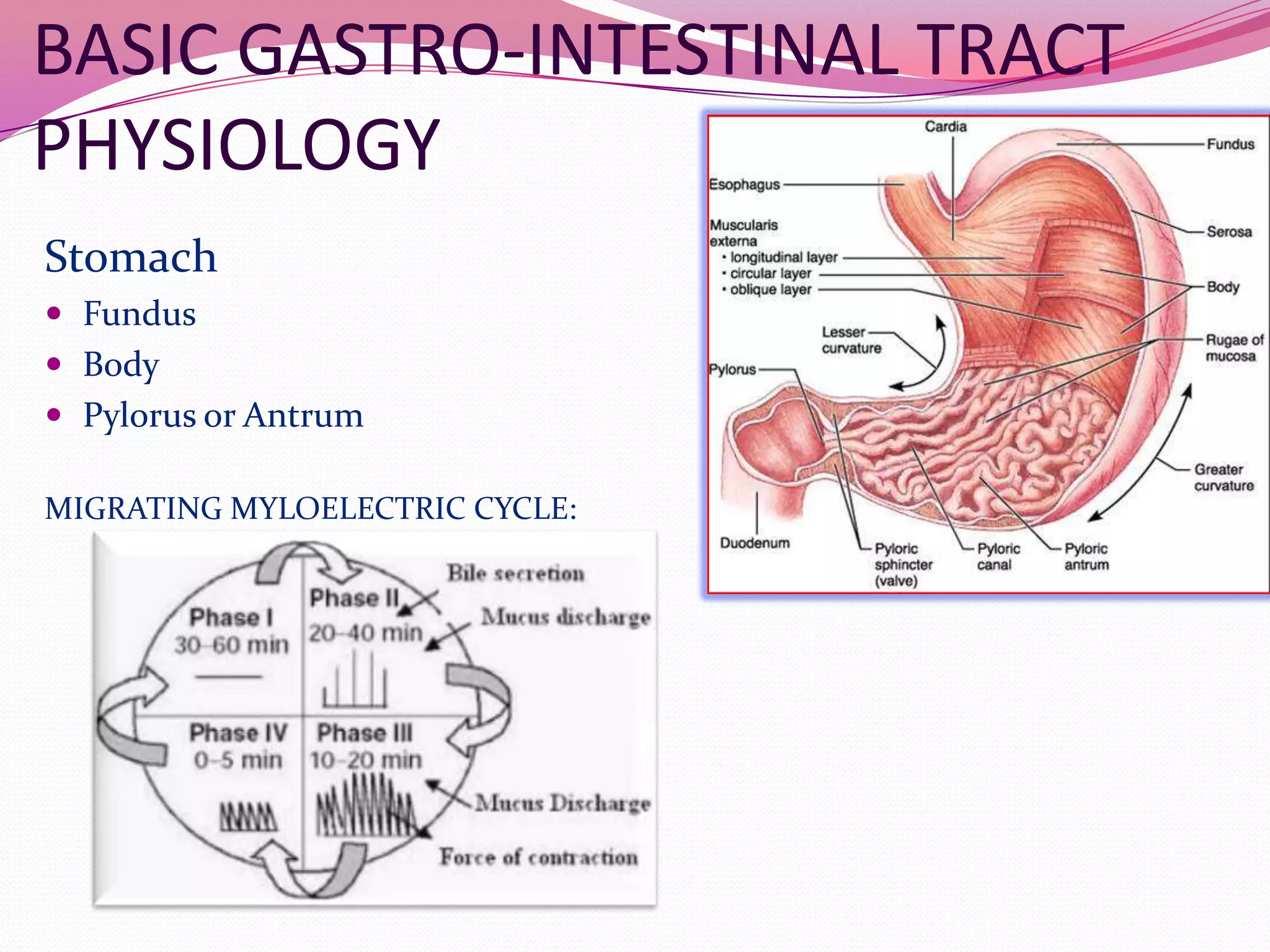
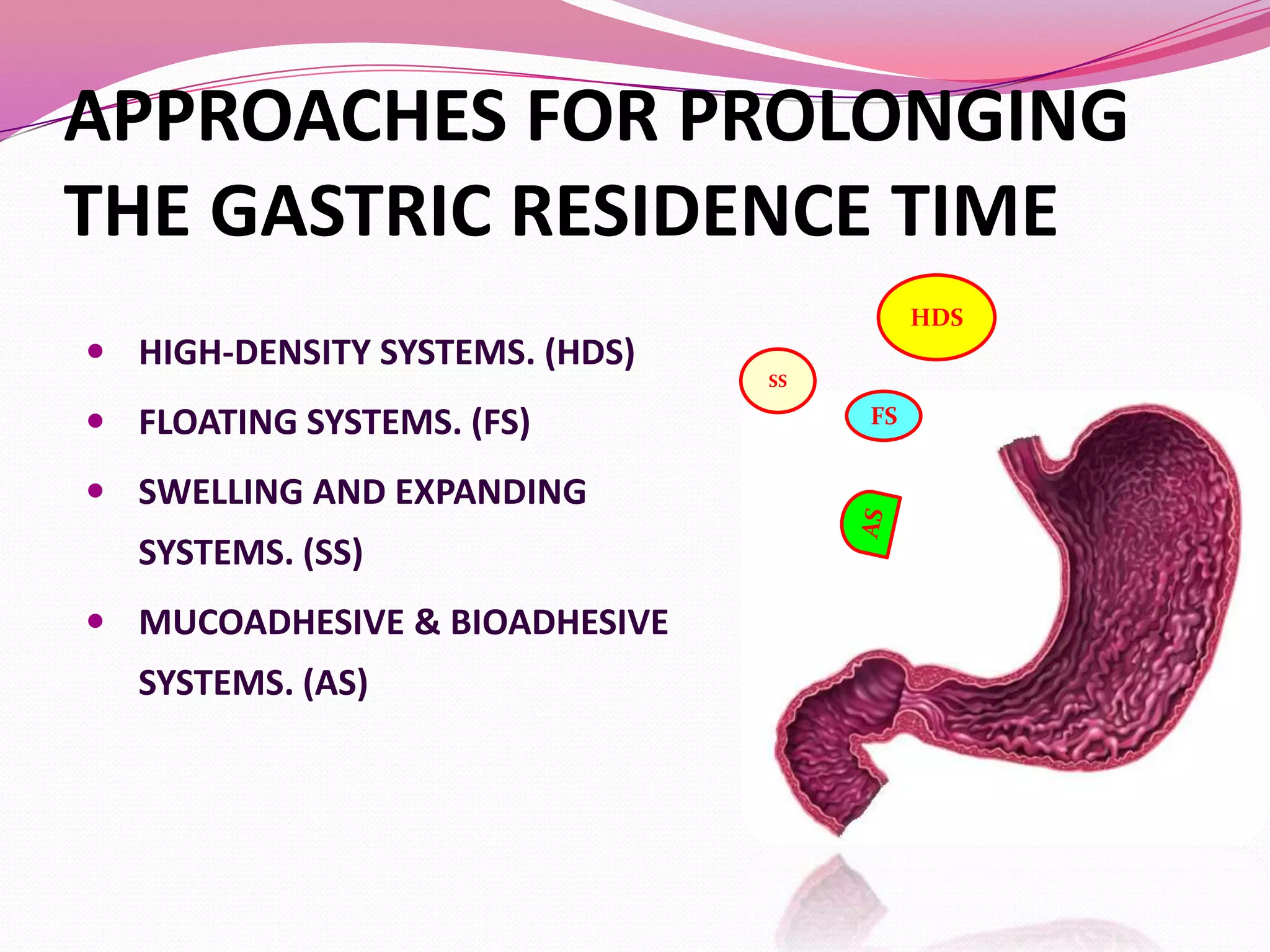
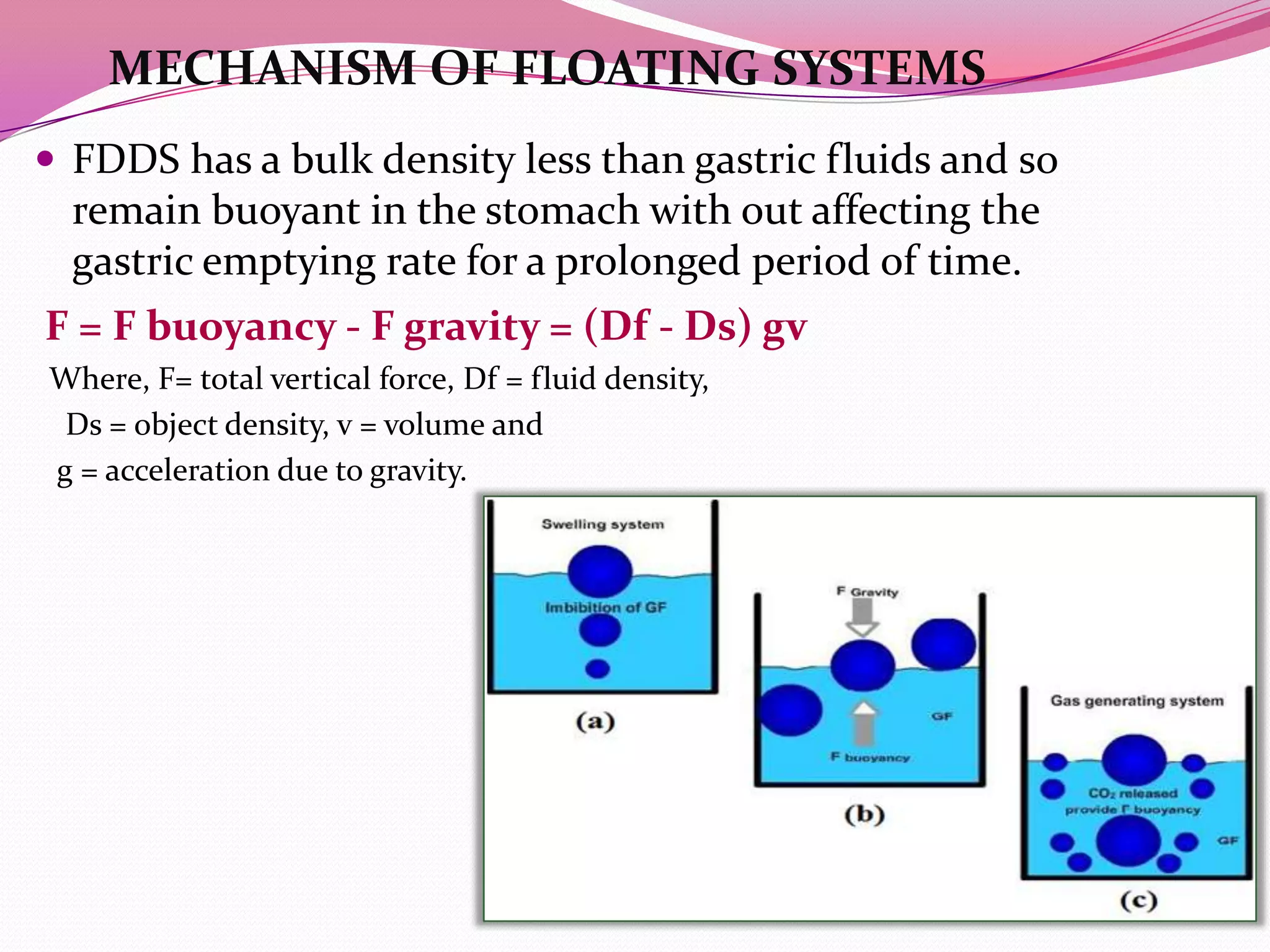
This document discusses floating drug delivery systems (FDDS). It begins with an introduction describing FDDS as low-density systems that remain buoyant in the stomach without affecting gastric emptying rate, resulting in increased gastric retention time. It then covers basic gastrointestinal tract physiology, approaches to prolonging gastric residence time including floating systems, and the mechanisms of floating systems. Some advantages include enhanced bioavailability and sustained drug delivery. Widely used polymers, preparation methods, classifications, evaluation tests, marketed products, and conclusions are also summarized.






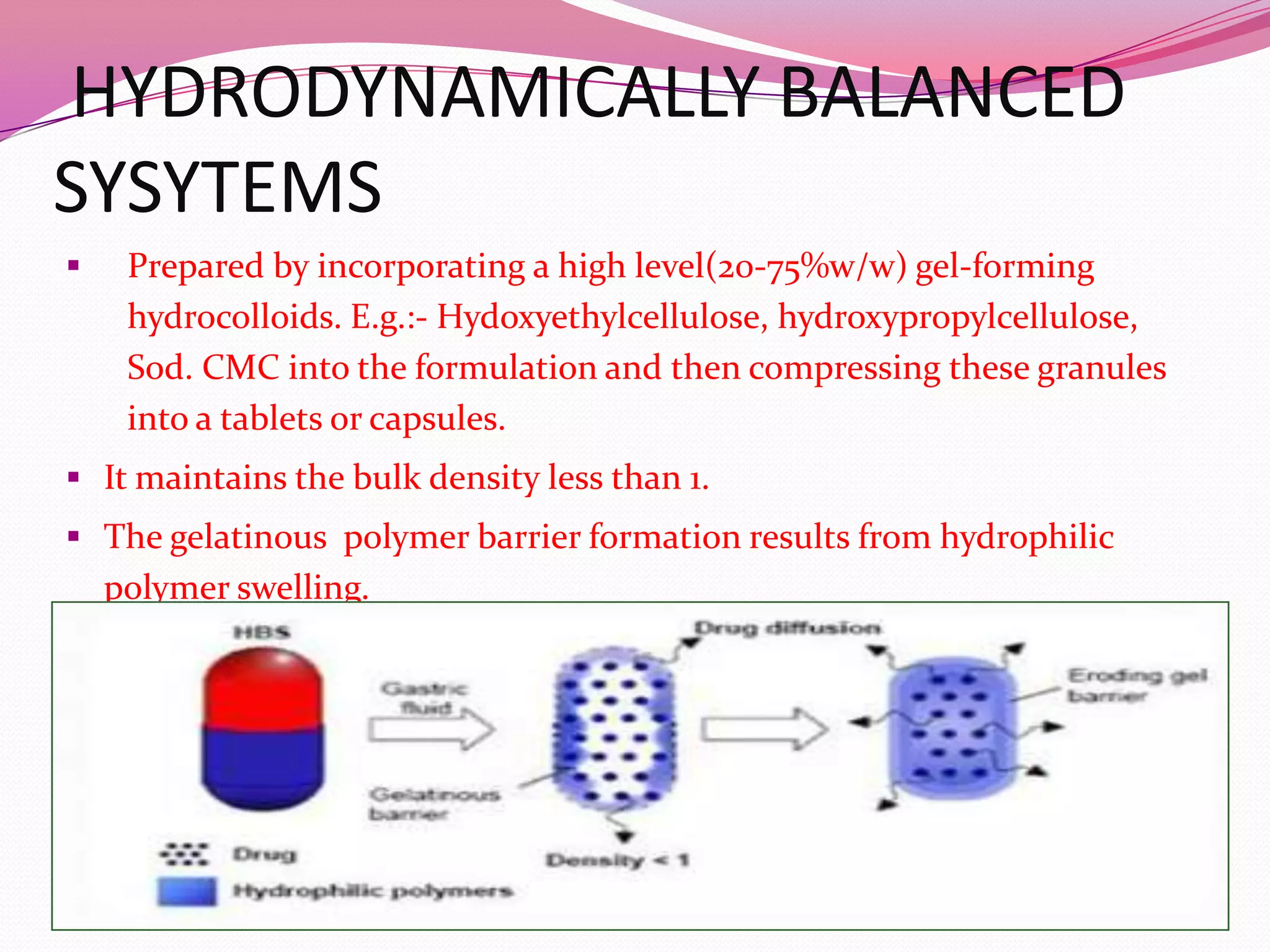
![Non effervescent systems: One or more gel forming, highly swellable,
cellulosic hydrocolloids (e.g. hydroxyl ethyl
cellulose, hydroxypropyl methyl cellulose
[HPMC] etc, polysaccharides, or matrix forming
polymers(e.g polyacrylates, and polystyrene) are
incorporated in high level (20‐75% w/w) to
tablets or capsules.](https://image.slidesharecdn.com/fddsppt-140121213908-phpapp02/75/Floating-drug-delivery-system-ppt-14-2048.jpg)