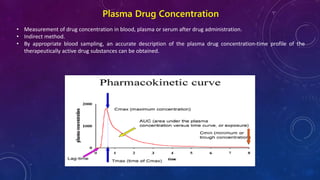
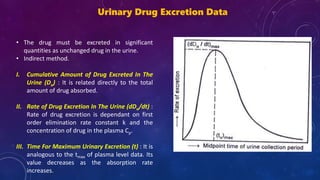
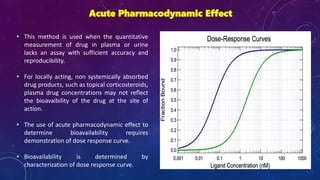
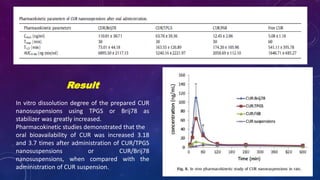
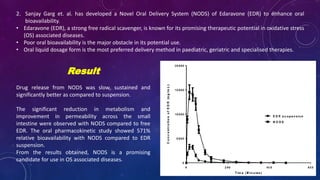
This document summarizes a seminar presentation on methods for determining bioavailability. It defines bioavailability as the rate and extent to which the active substance of a drug is absorbed and available at the site of action. It then describes the main objectives of bioavailability studies which include aiding new drug and formulation development. The key methods discussed for assessing bioavailability include measuring plasma drug concentration, urinary drug excretion, acute pharmacodynamic effects, clinical observations, and in vitro drug dissolution studies. Specific parameters are defined for each method such as Cmax, AUC, tmax, Du, and Emax. Finally, the document summarizes two literature articles that developed formulations to enhance the oral bioavailability of curcumin and edarav