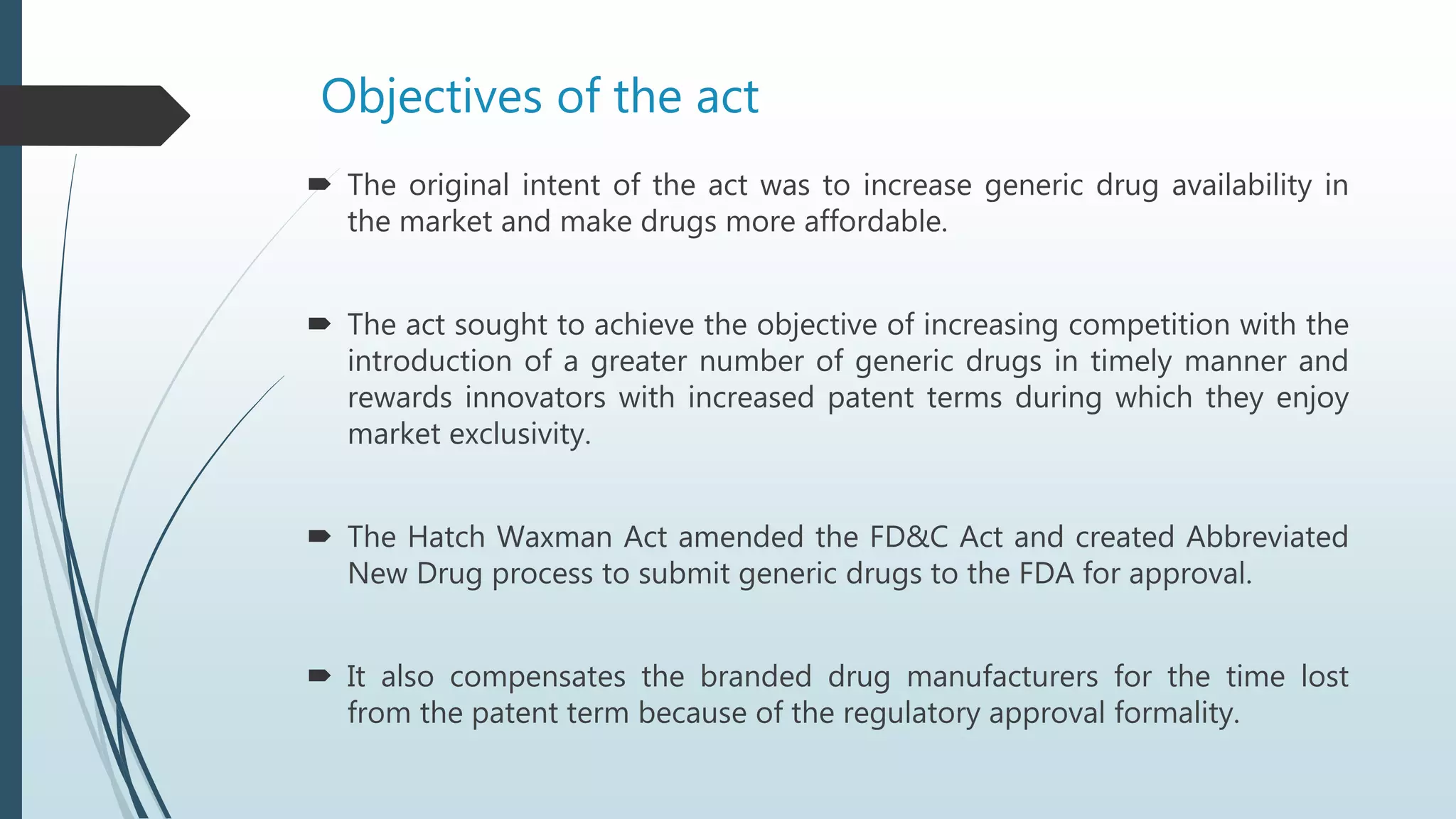
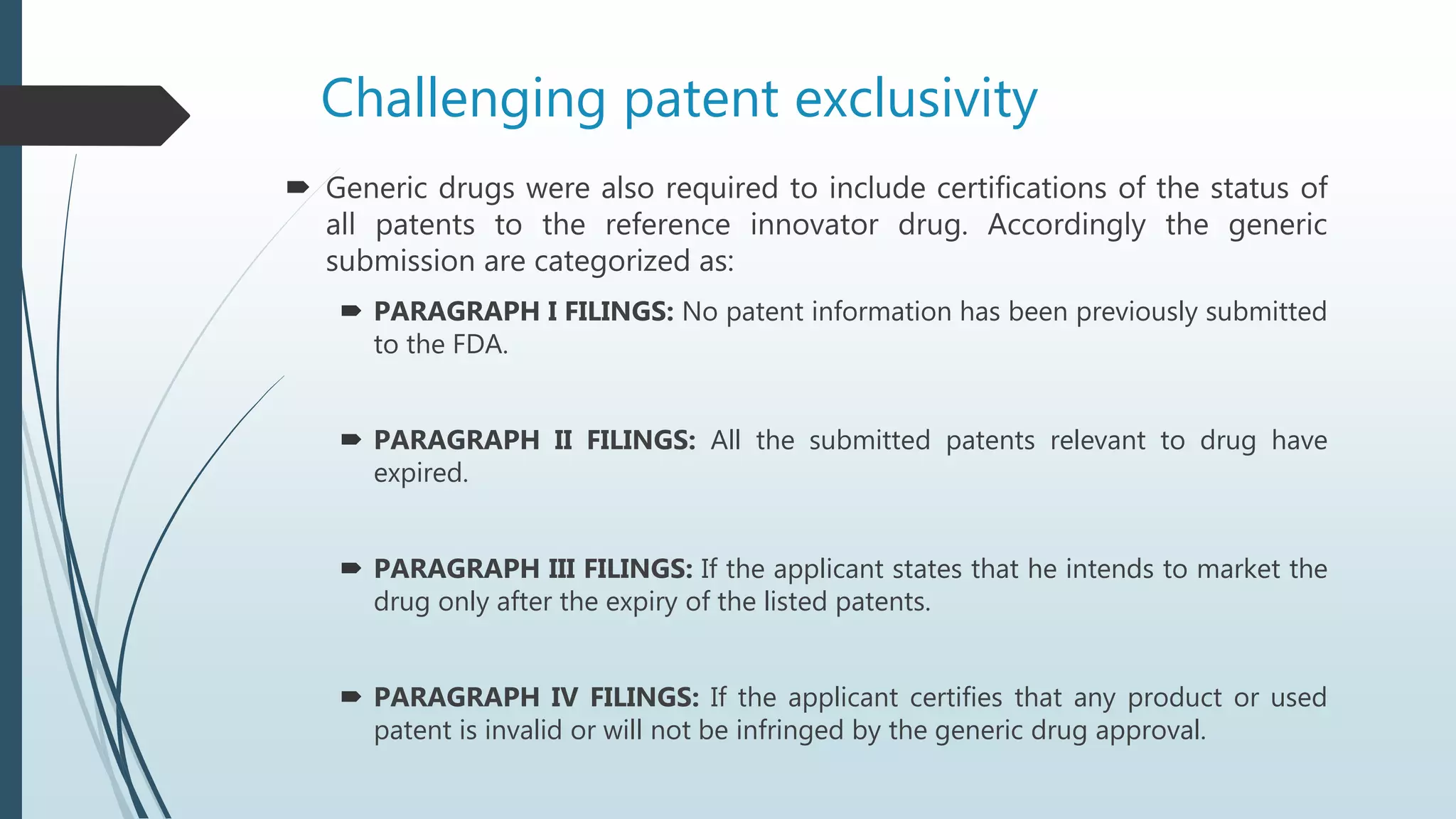
The Hatch-Waxman Act established regulations for generic drugs in the US to facilitate generic competition. It created an Abbreviated New Drug Application process for generics to gain approval more quickly by showing bioequivalence to branded drugs. The first generic to challenge a patent via Paragraph IV certification gains 180 days of marketing exclusivity. The Act also allows patent term extensions for branded drugs to compensate for regulatory approval times. While increasing generic competition, it provides benefits to branded manufacturers such as data and market exclusivities as well as patent dispute resolutions.