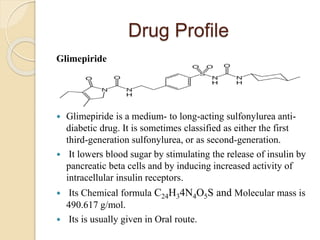
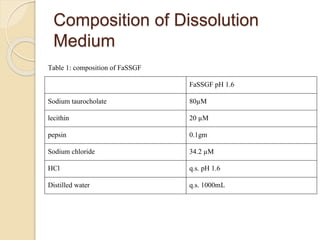
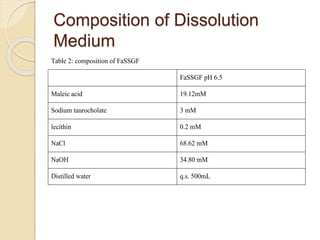
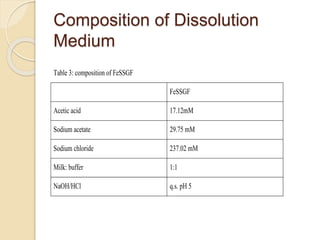
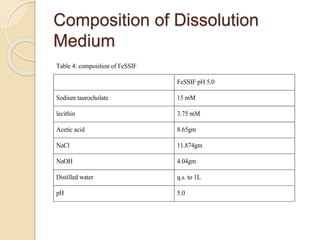
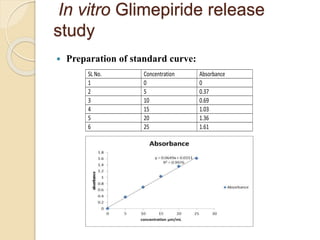
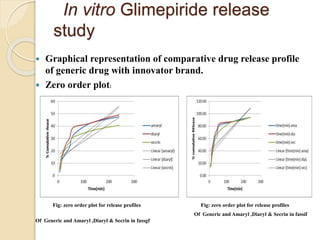
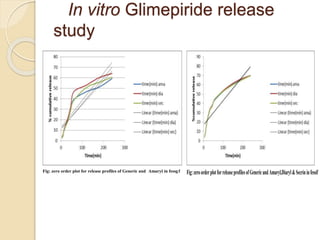
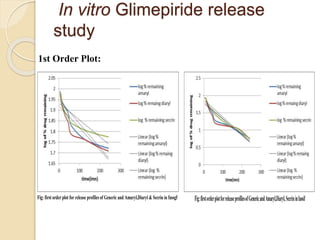
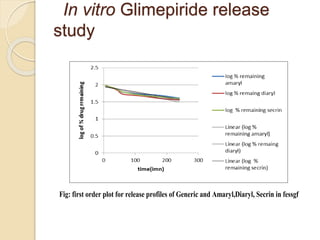
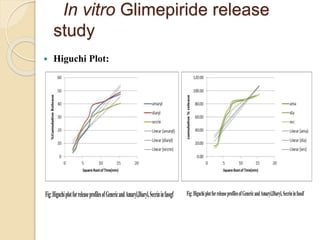
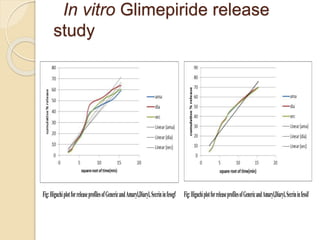
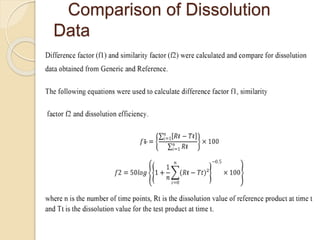
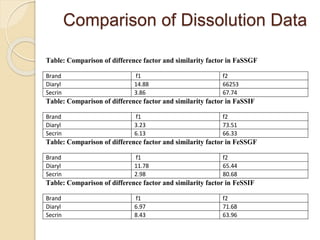
The document describes an in vitro bioequivalence study of Glimepiride using biorelevant media. The aim was to evaluate the dissolution rate of Glimepiride in different biorelevant media, compare the bioequivalence of local generic brands to an innovator brand, and predict the effect of food in the GI tract on dissolution. Dissolution studies were conducted in four biorelevant media with two tablets each of three brands. Results showed that dissolution was highest in the intestinal medium and reduced with food. Difference and similarity factors indicated the two generic brands were equivalent to the innovator brand. Using biorelevant media allowed prediction of dissolution rates and bioequivalence of the Glimepiride brands.