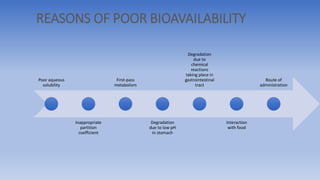
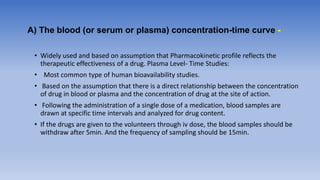
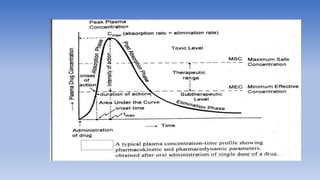
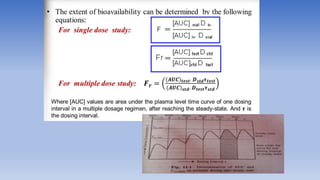
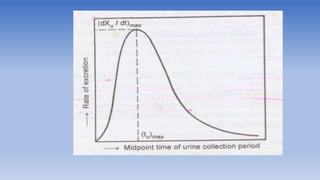
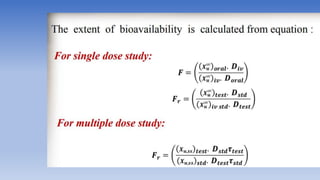
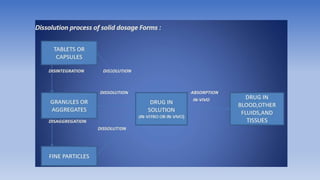
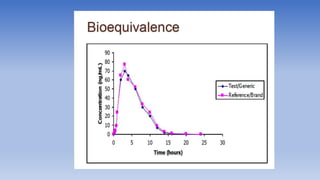
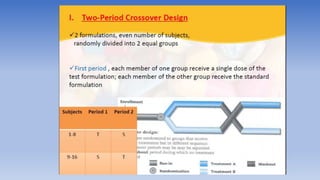
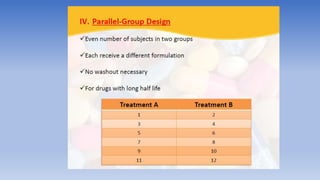
The document discusses bioavailability and bioequivalence, focusing on the extent and rate at which a drug enters systemic circulation and the importance of ensuring that the delivered dose is effective. It outlines objectives for bioavailability studies, methods for improvement, and different assessment techniques, including pharmacokinetic and pharmacodynamic methods. Additionally, it covers in vitro dissolution testing and the conditions under which bioequivalence can be established, particularly regarding generic drugs.