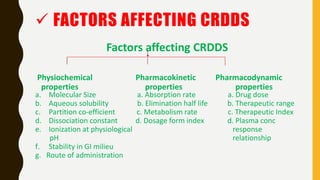
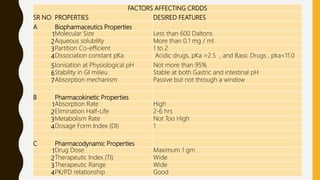
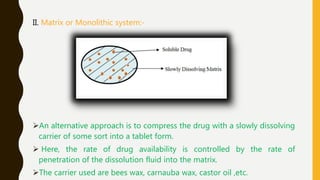
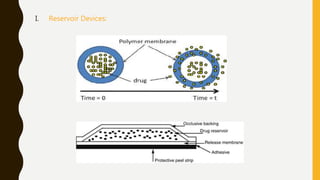
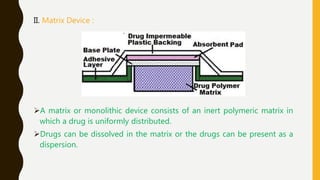
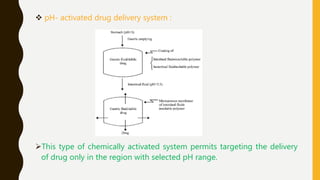
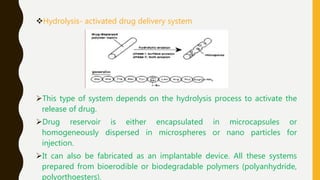
This document provides an overview of controlled release drug delivery systems (CRDDS). It defines CRDDS as systems that provide some control over the temporal or spatial release of drugs. The key advantages of CRDDS are maintaining effective drug levels, decreasing dosing frequency and side effects, and improving patient compliance. Factors like drug properties, pharmacokinetics, and pharmacodynamics can affect CRDDS. Various approaches to designing CRDDS are discussed.