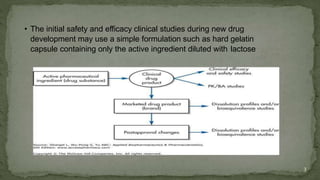
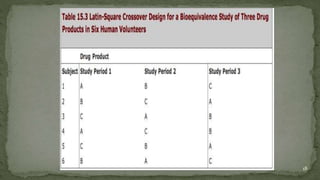
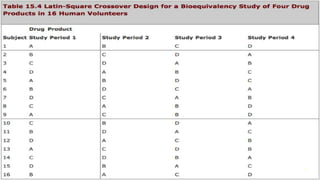
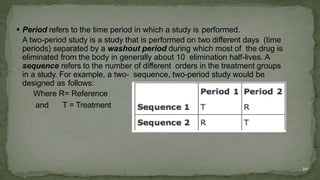
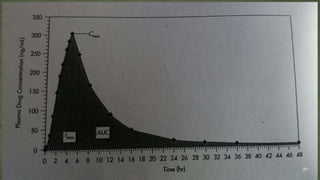
The document discusses drug product performance and in vivo bioavailability and bioequivalence. It defines bioavailability as the release of a drug substance from a product leading to absorption. Bioavailability studies are used to understand how formulation changes impact pharmacokinetics. They are important for developing new and generic drug products. The document outlines study designs like single-dose fasting studies and food effect studies used to assess bioavailability and establish bioequivalence between products. It also discusses key pharmacokinetic parameters measured in such studies like Cmax, Tmax, and AUC.