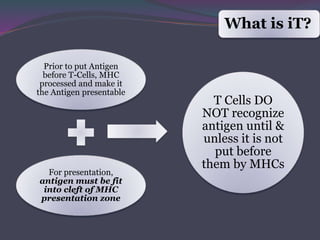
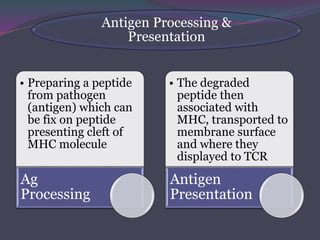
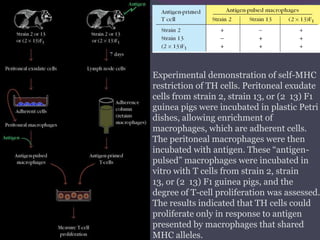
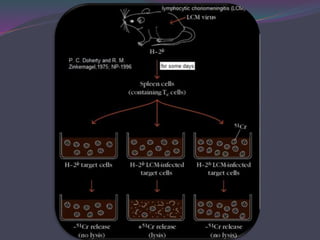
1. Antigen processing and presentation involves degradation of antigens into peptides, association of peptides with MHC molecules, and display of peptide-MHC complexes on the cell surface for recognition by T cells.
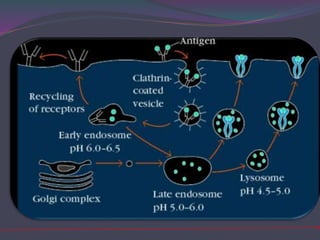
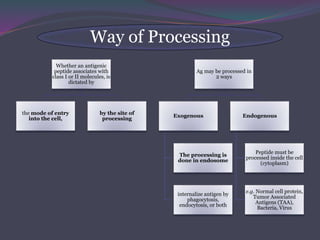
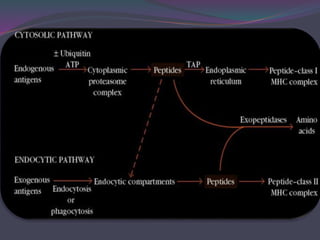
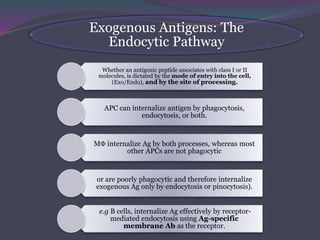
2. There are two main pathways of antigen processing - exogenous antigens that enter the cell are processed through the endocytic pathway while endogenous antigens are processed through the cytosolic pathway.
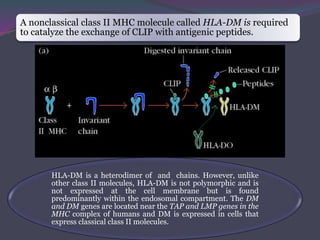
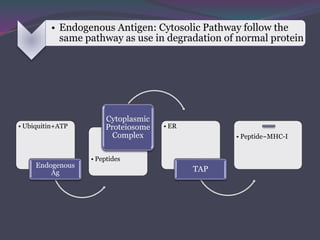
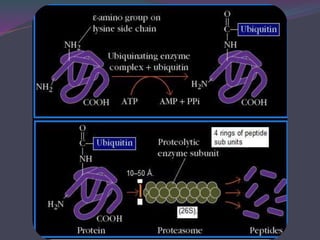
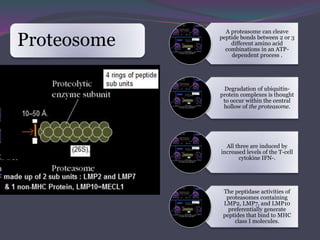
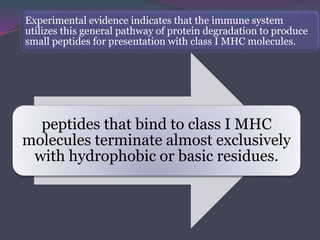
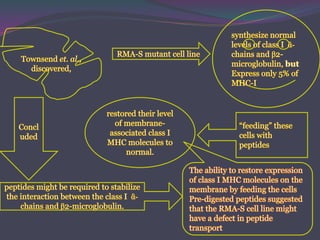
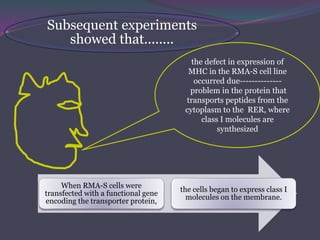
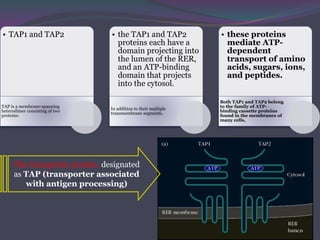
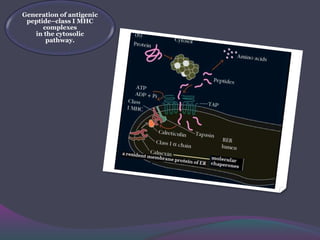
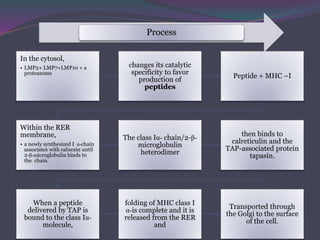
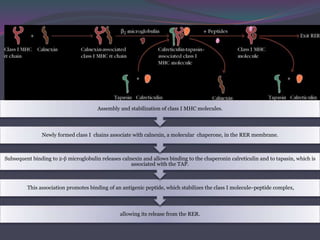
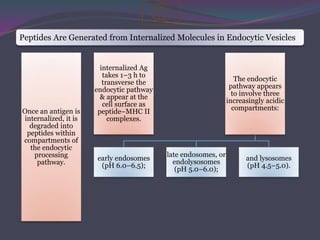
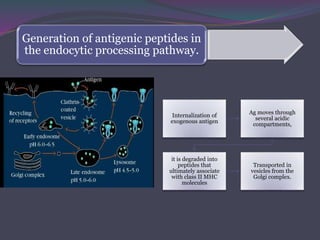
3. In the cytosolic pathway, antigens are degraded by the proteasome and transported by TAP into the ER where they can bind to MHC class I molecules. In the endocytic pathway, exogenous antigens internalized into vesicles are degraded into peptides that bind MHC class II molecules.
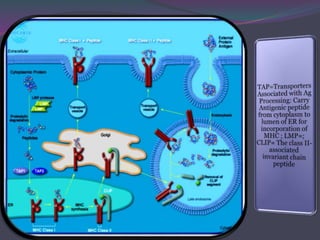
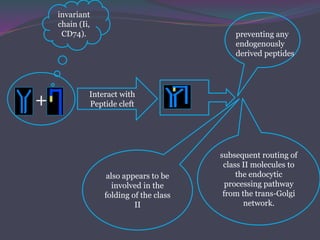
![The Invariant Chain [Ii]Guides Transport of Class II MHC
Molecules to Endocytic Vesicles
Since antigen-presenting
cells express both class I
and class II MHC
molecules,
Some mechanism must
exist to prevent class II
MHC molecules from
binding to the same set of
antigenic peptides as the
class I molecules.
When class II MHC
molecule are
synthesized within
the RER, 3 pairs of
class II chains
associate with a
preassembled trimer
of a protein called
invariant chain (Ii,
CD74).](https://image.slidesharecdn.com/antigenprocessingpresentation-150831063300-lva1-app6891/85/Antigen-processing-presentation-22-320.jpg)