1. The document discusses the two pathways that the immune system uses to process endogenous and exogenous antigens for presentation to T cells.
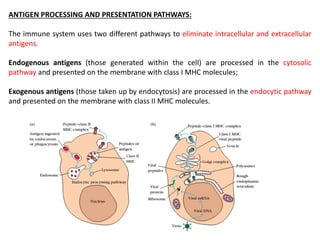
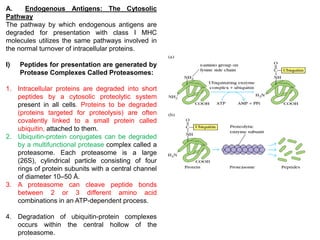
2. The cytosolic pathway processes endogenous antigens within the cytoplasm, where they are degraded by proteasomes and transported by TAP proteins to associate with class I MHC molecules in the ER.
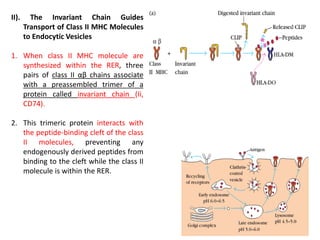
3. The endocytic pathway processes exogenous antigens that are taken up by endocytosis, degraded within acidic endosomes and lysosomes, and associate with class II MHC molecules aided by the invariant chain and HLA-DM/DO proteins.