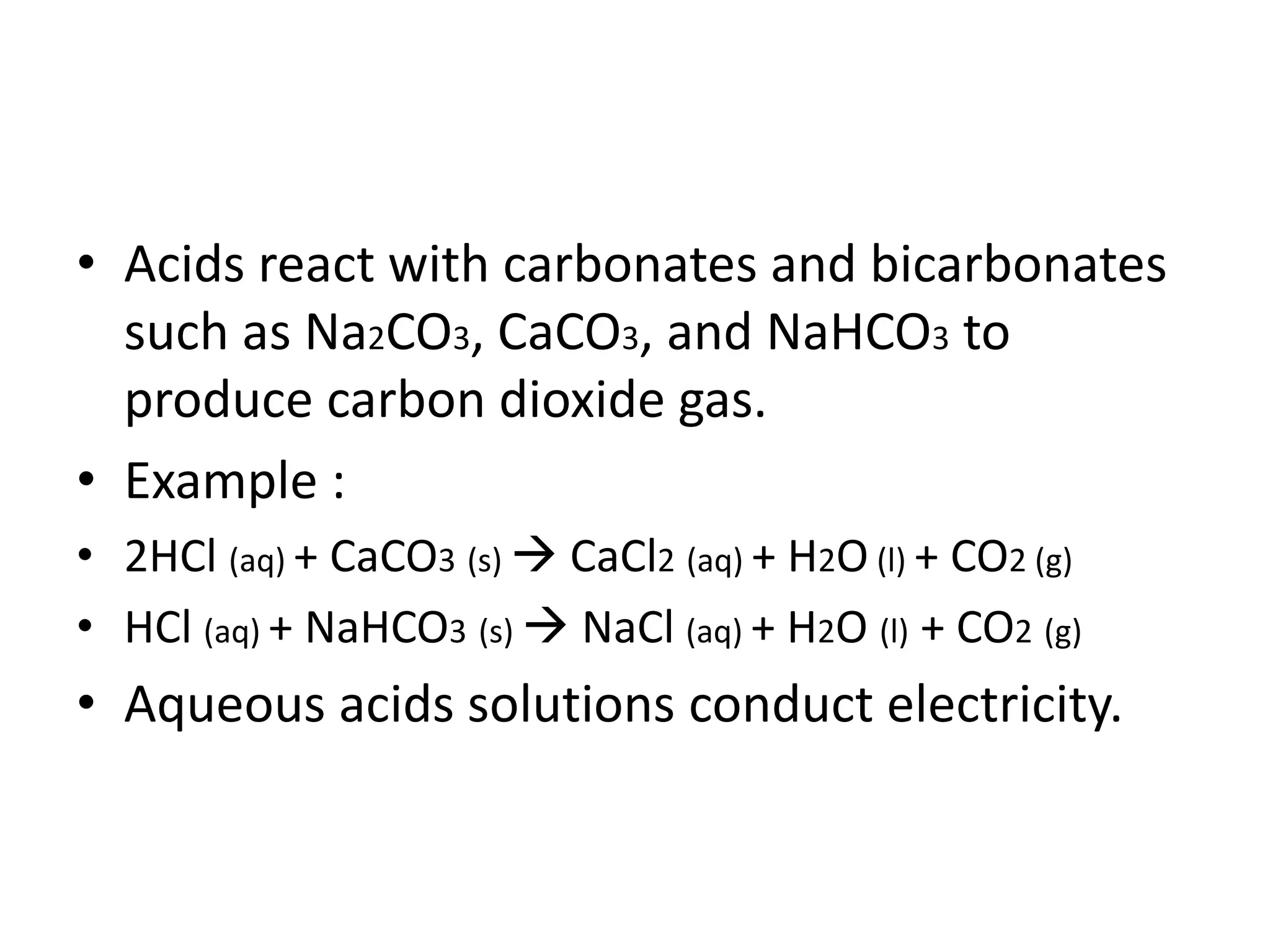
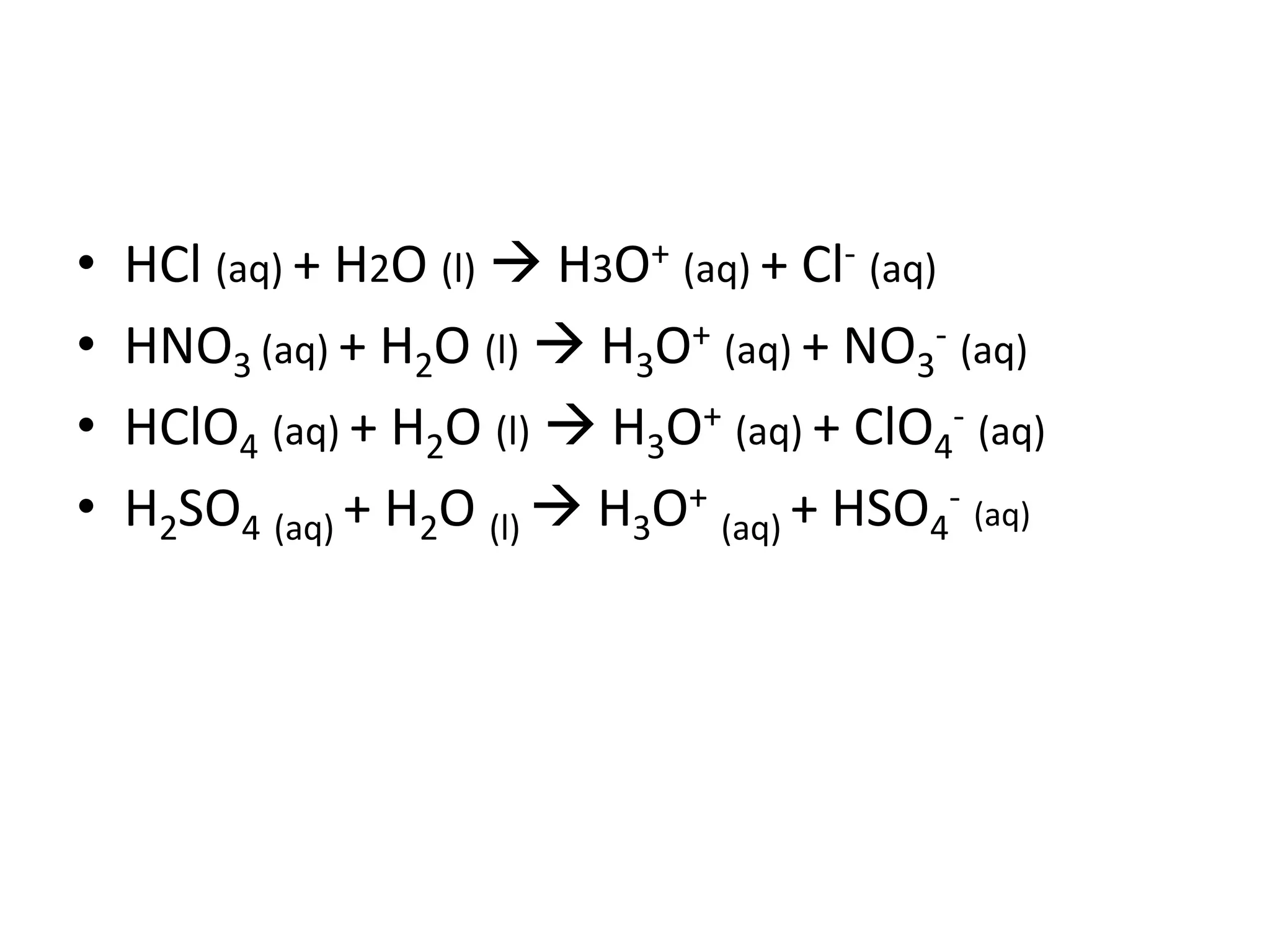
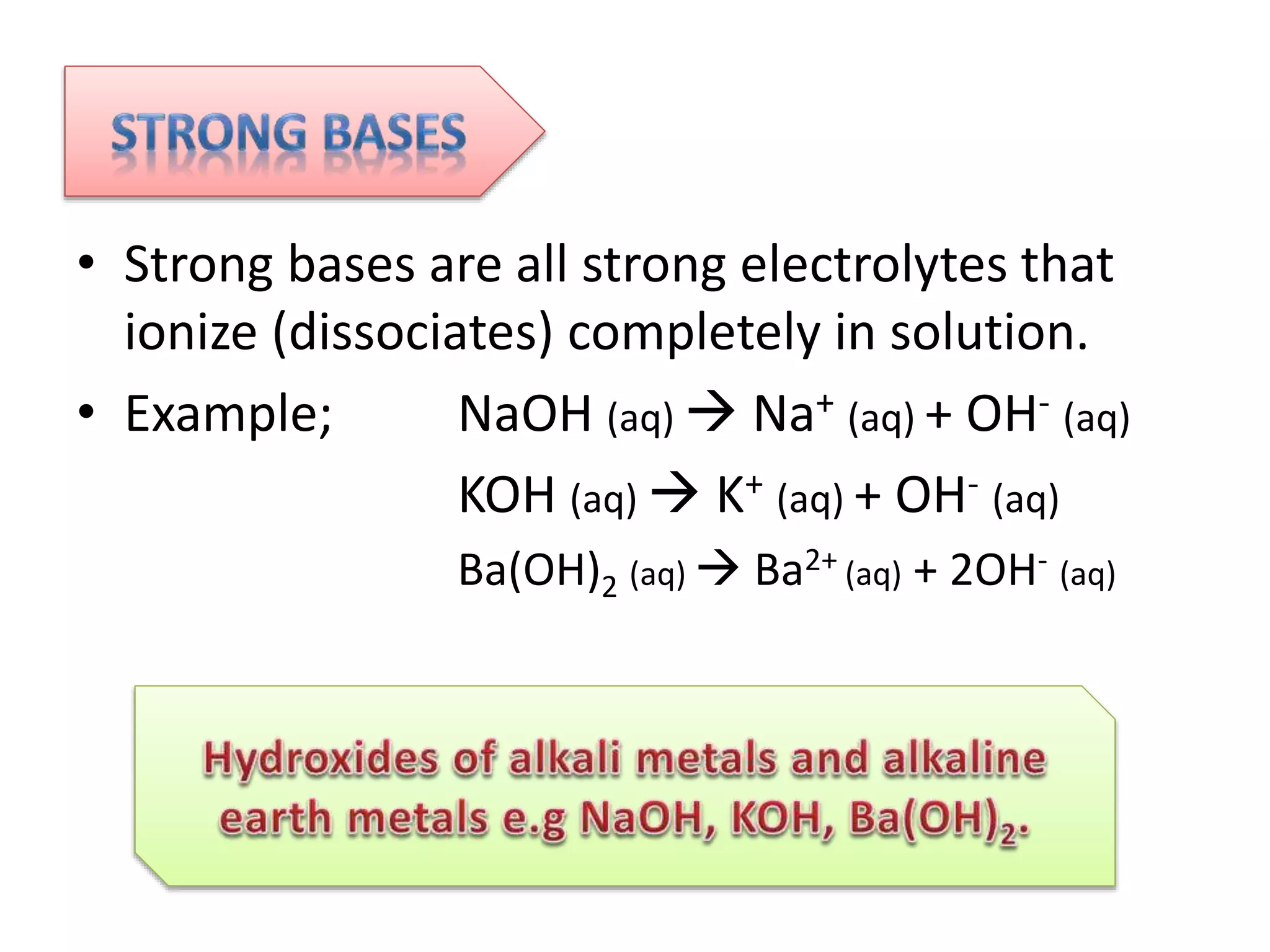
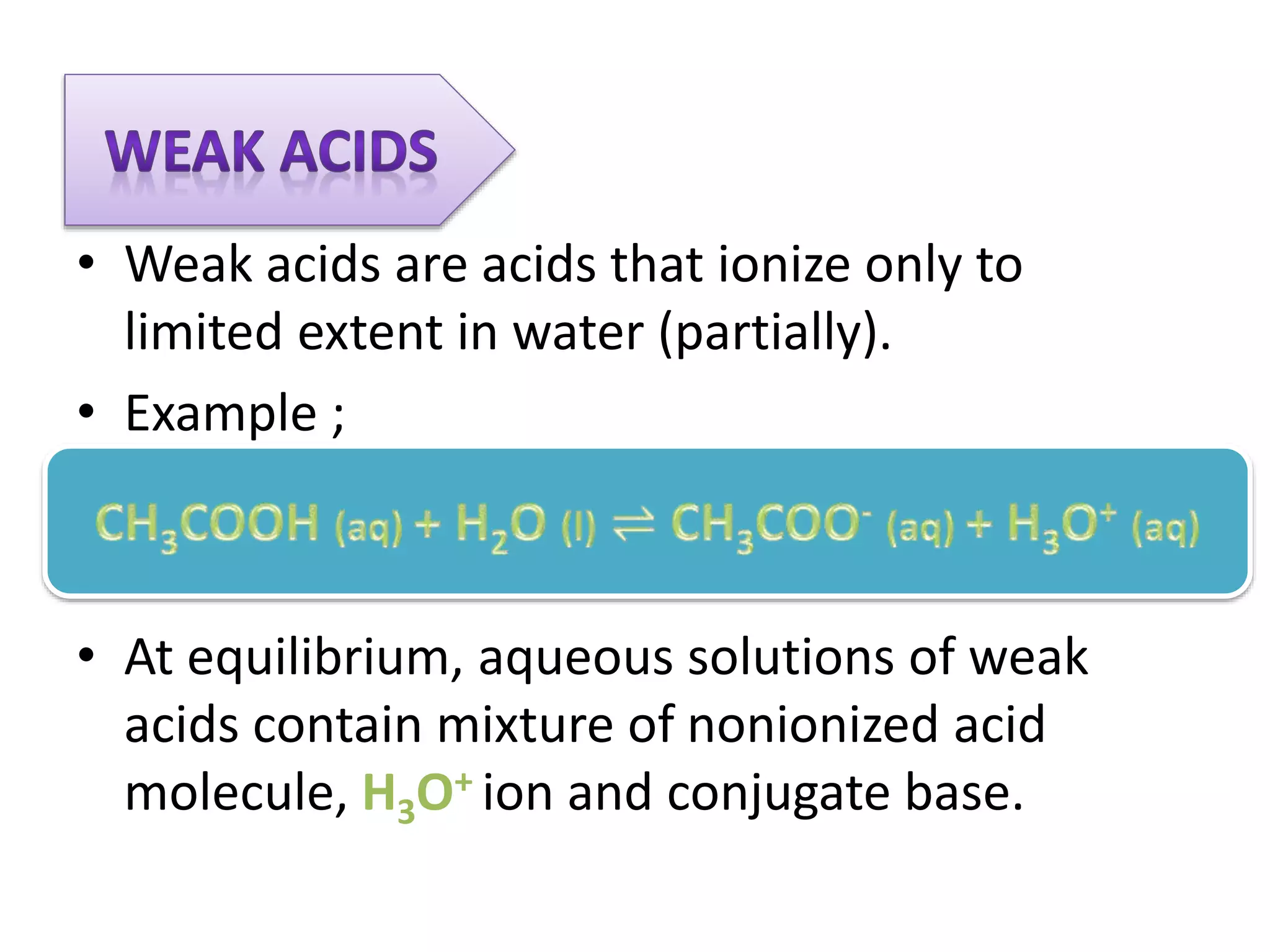
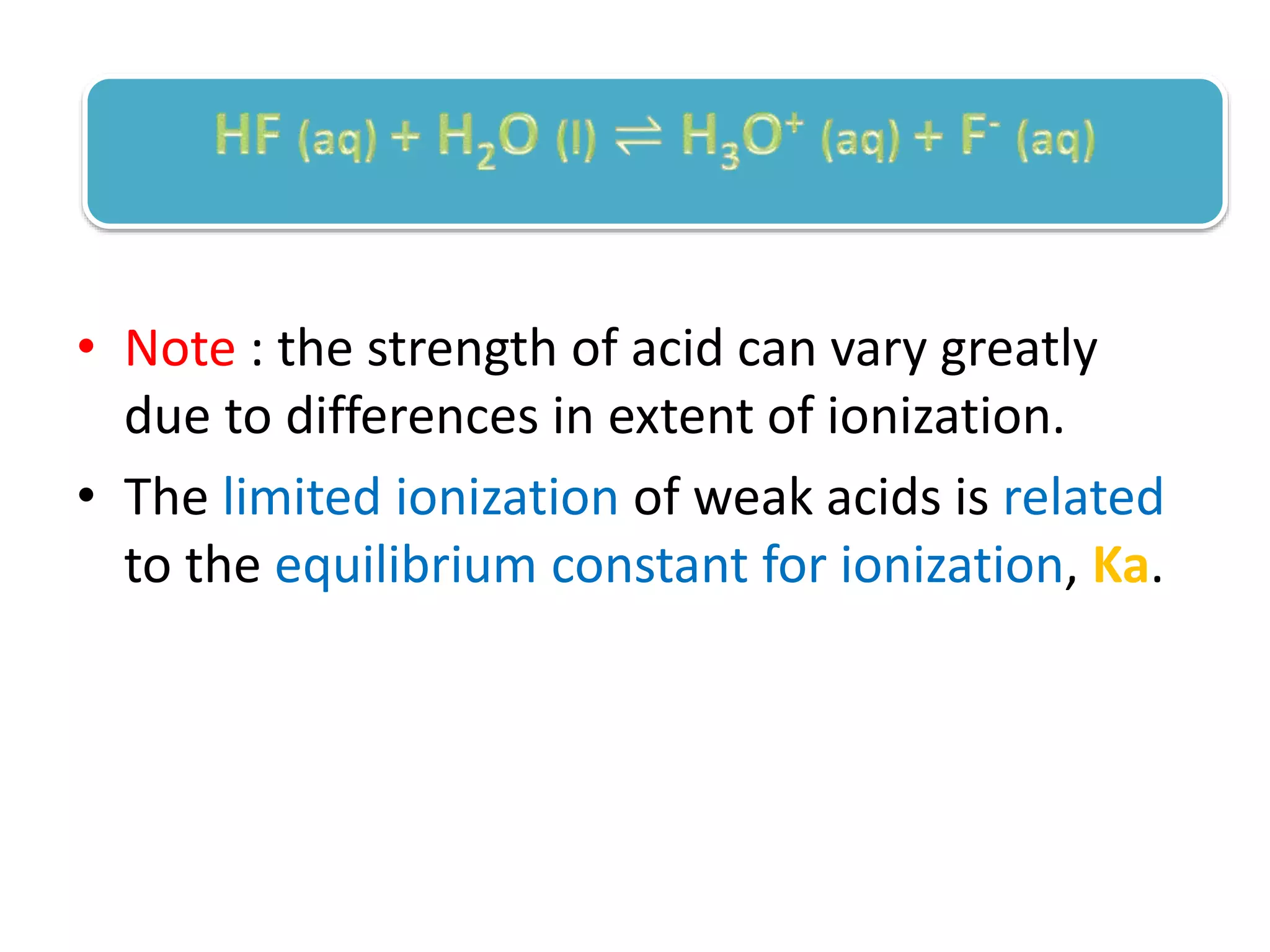
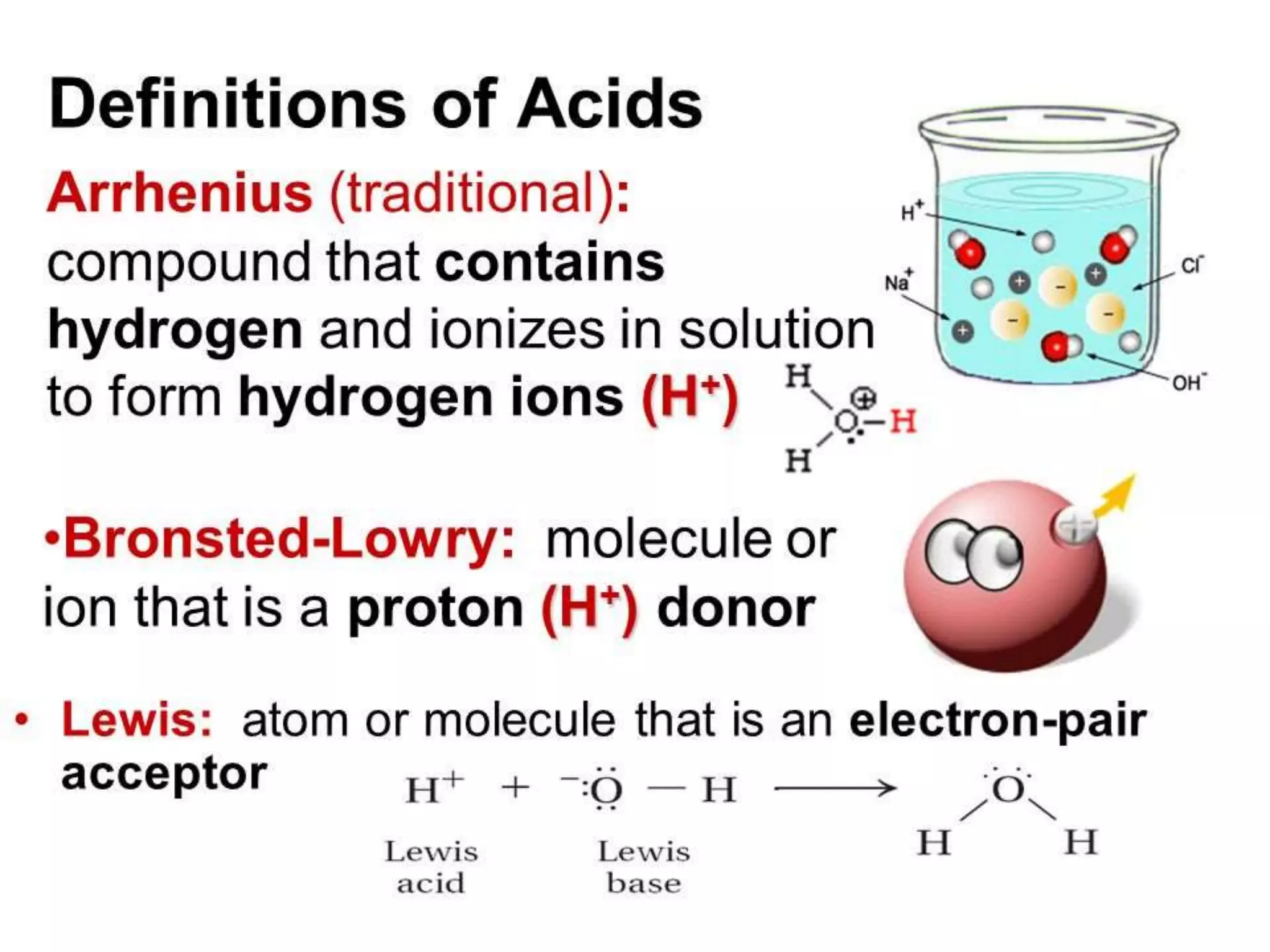
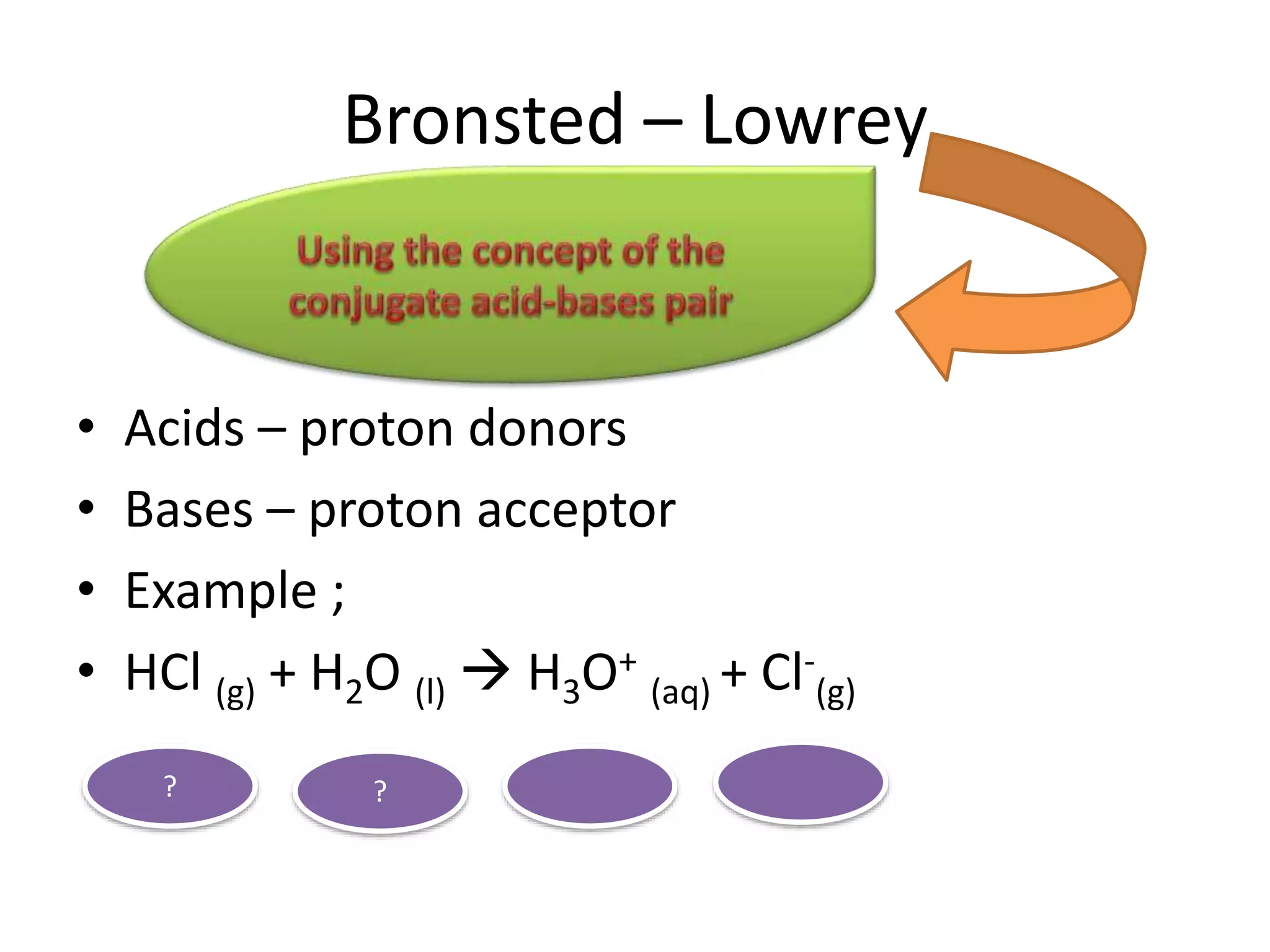
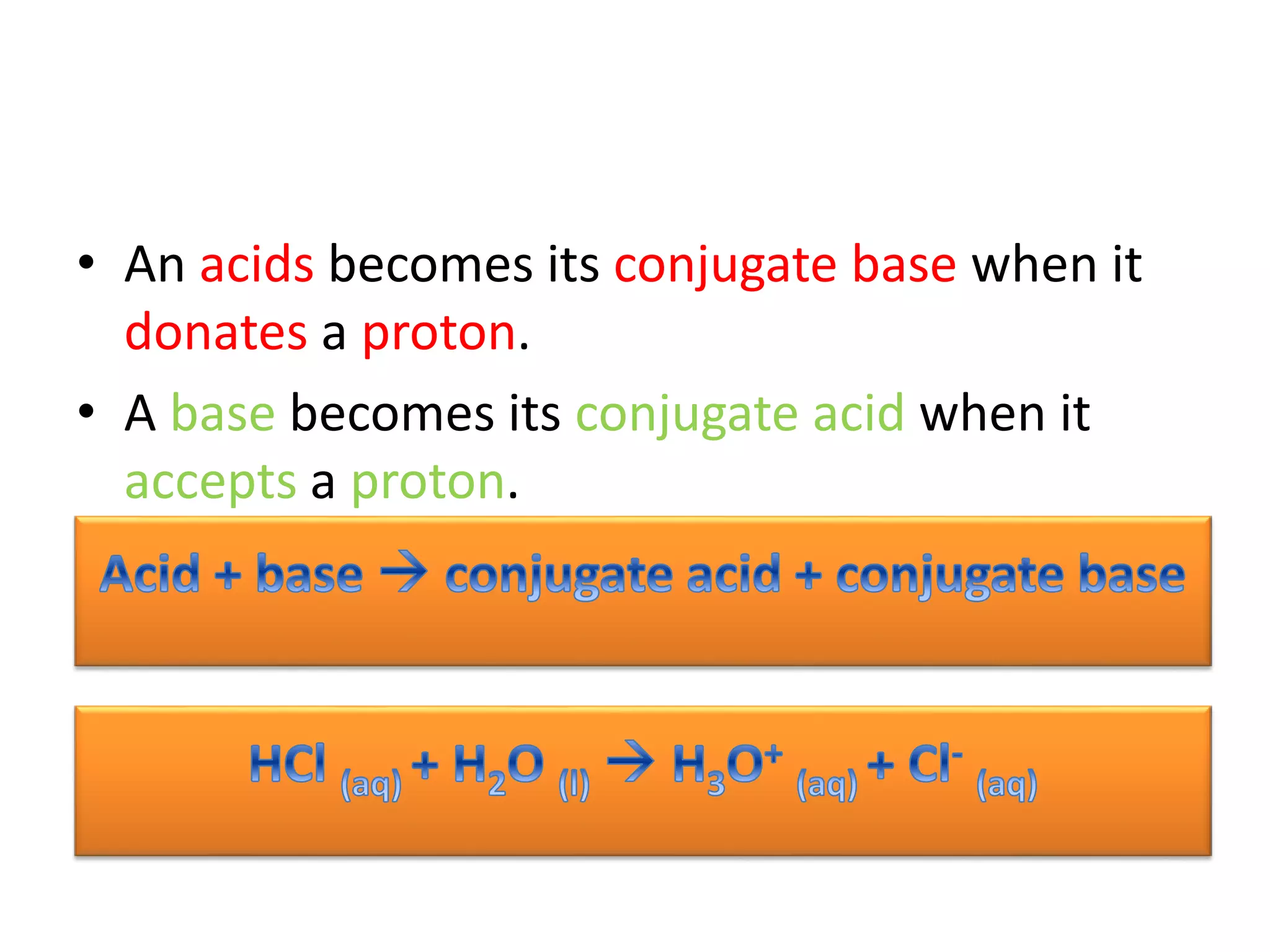
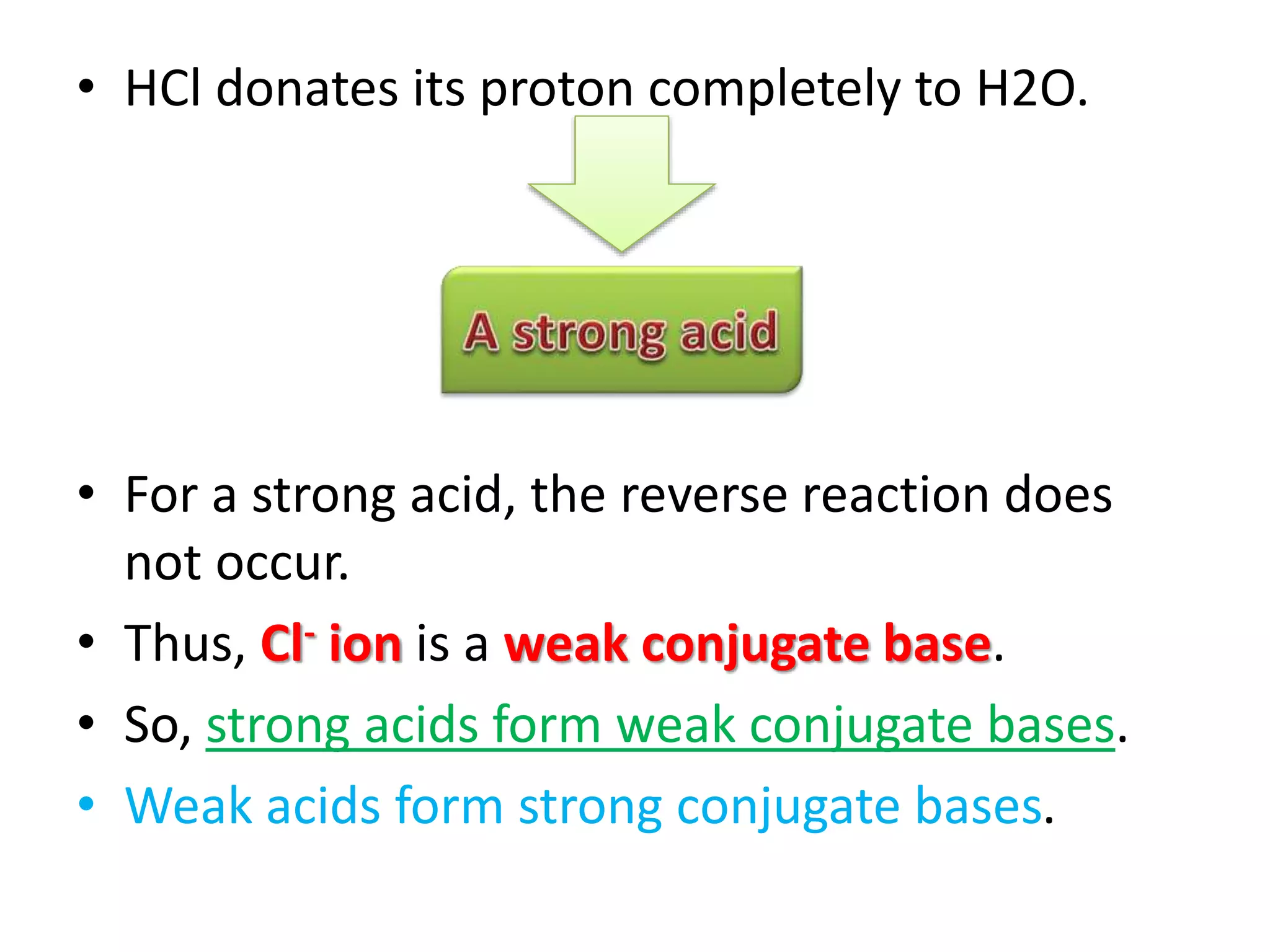
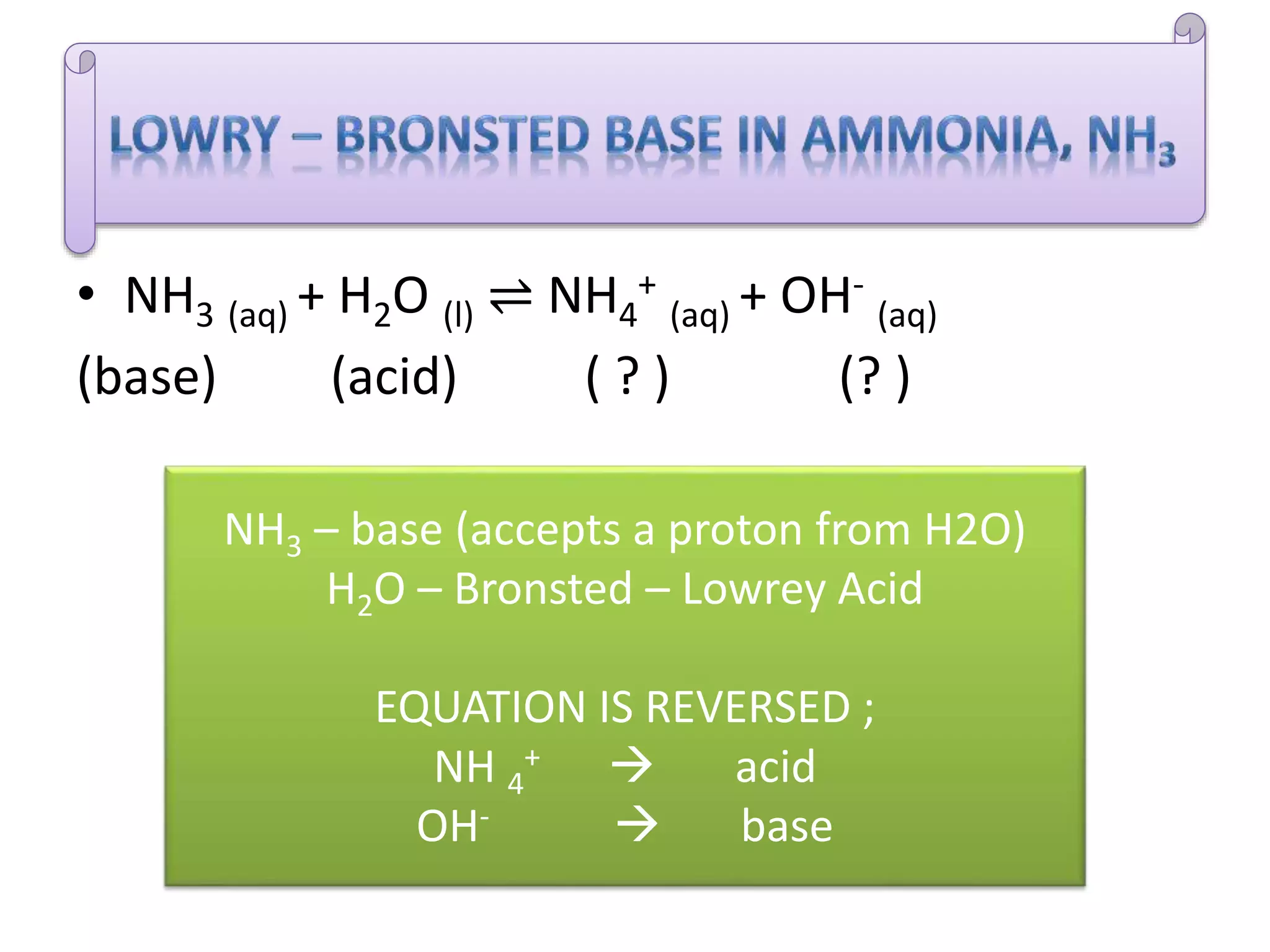
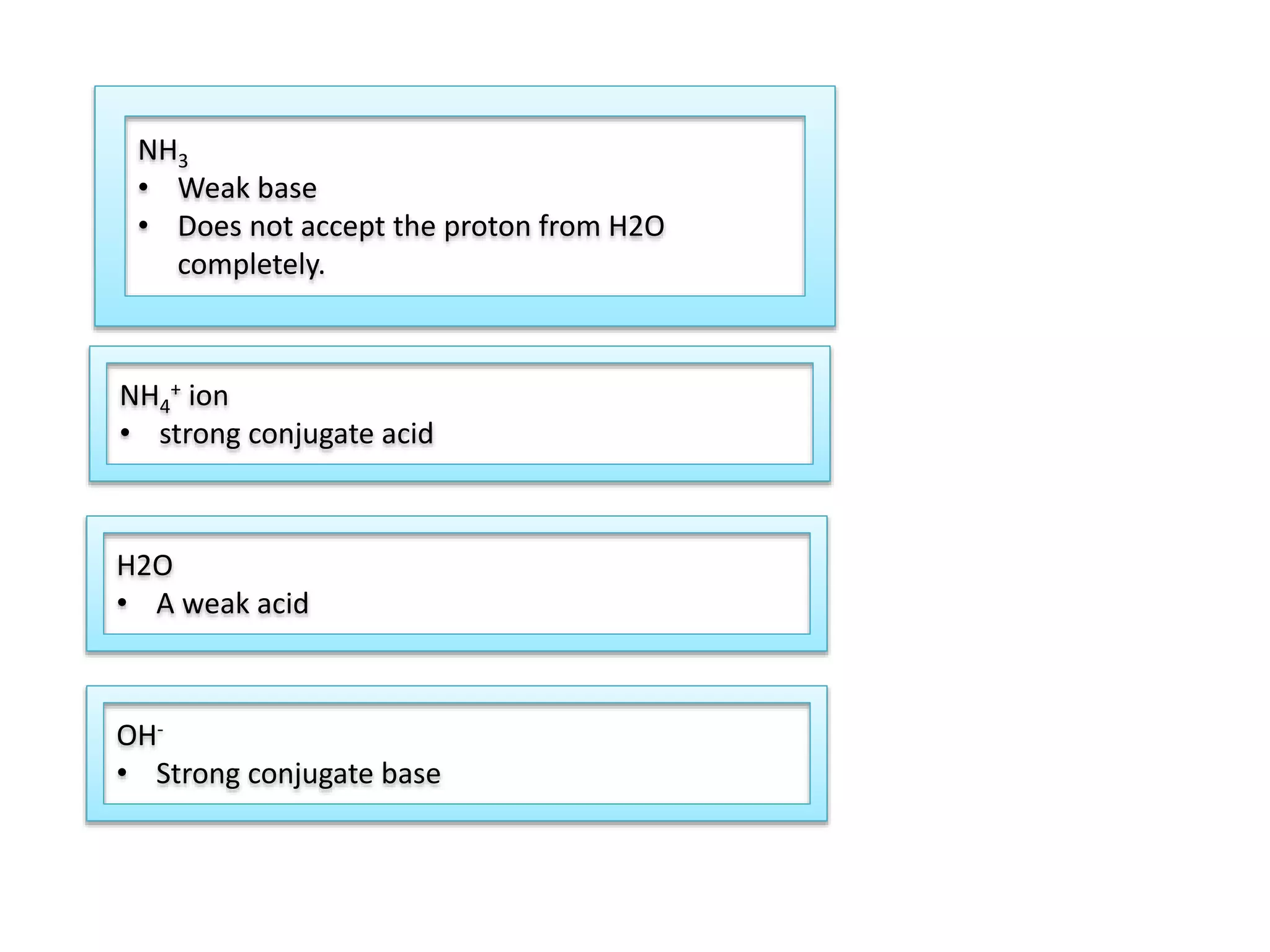
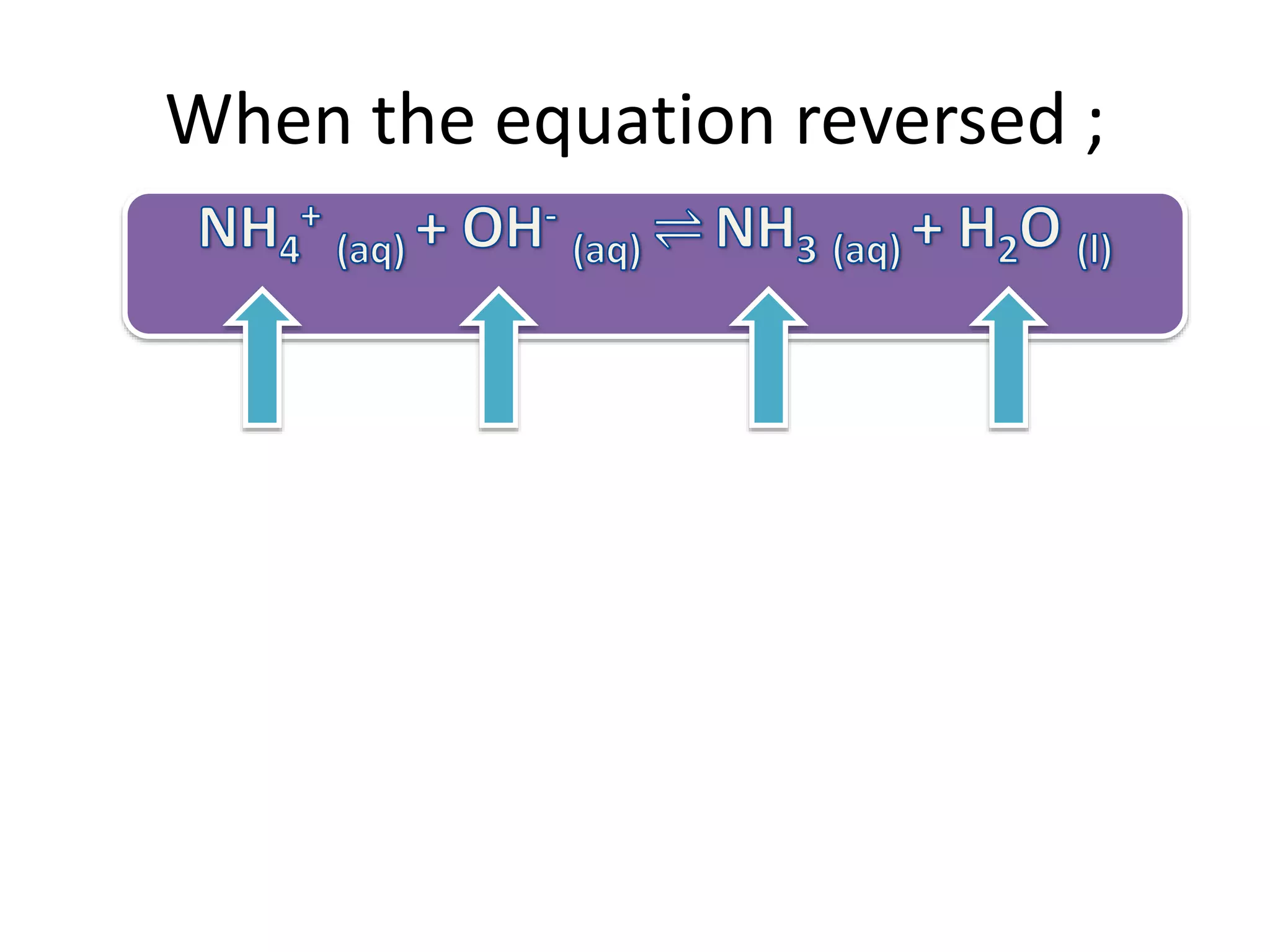
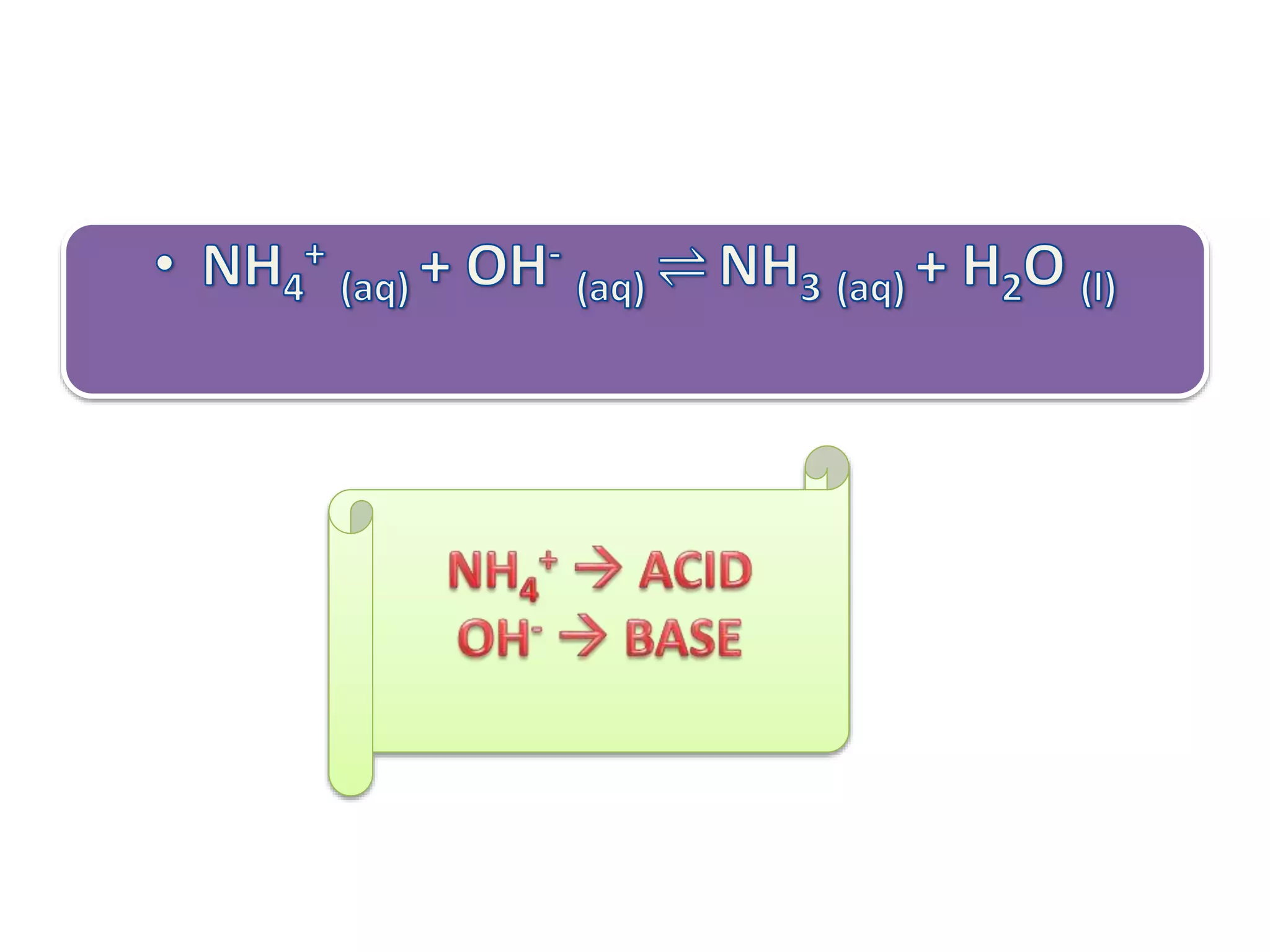
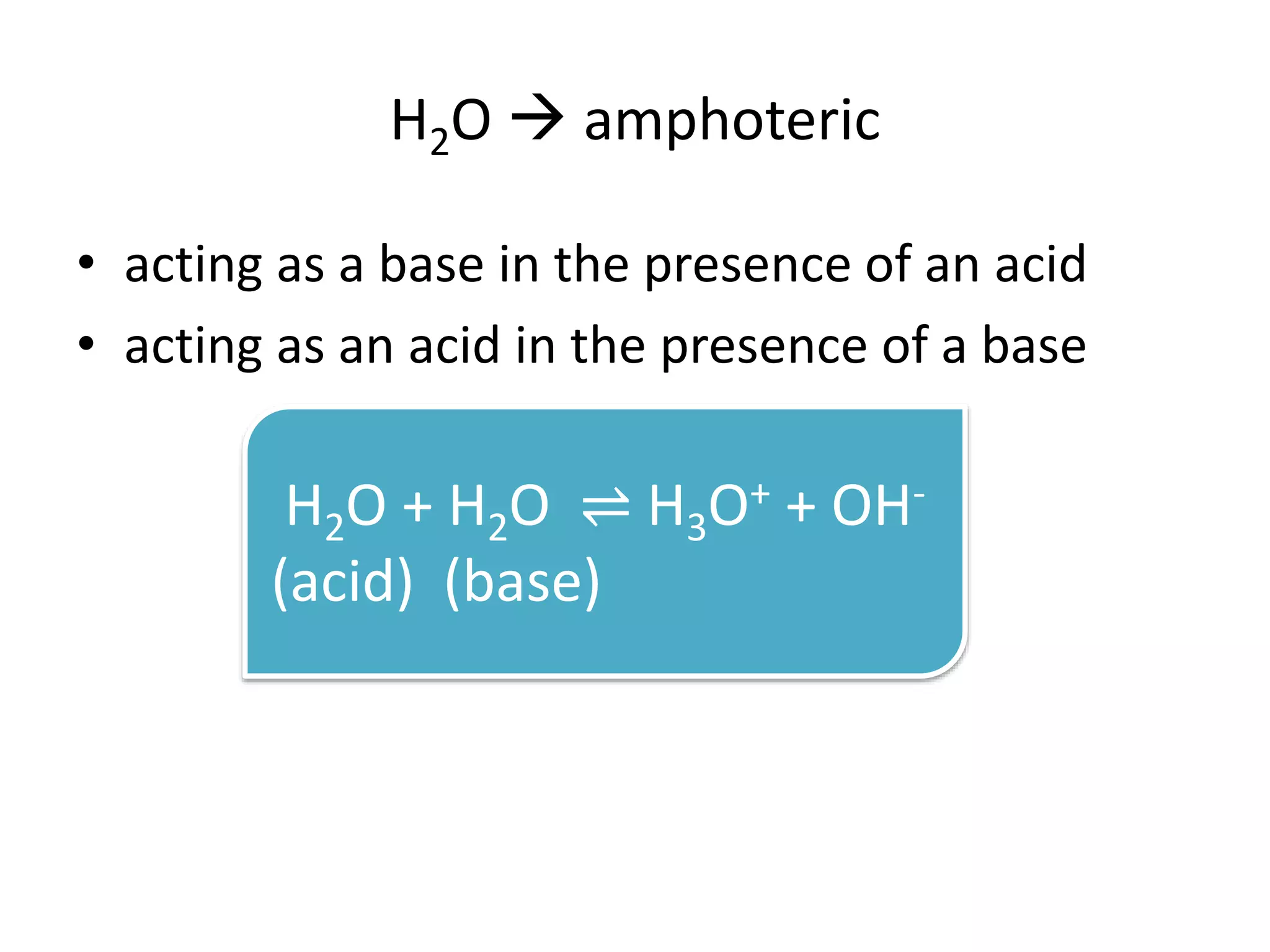
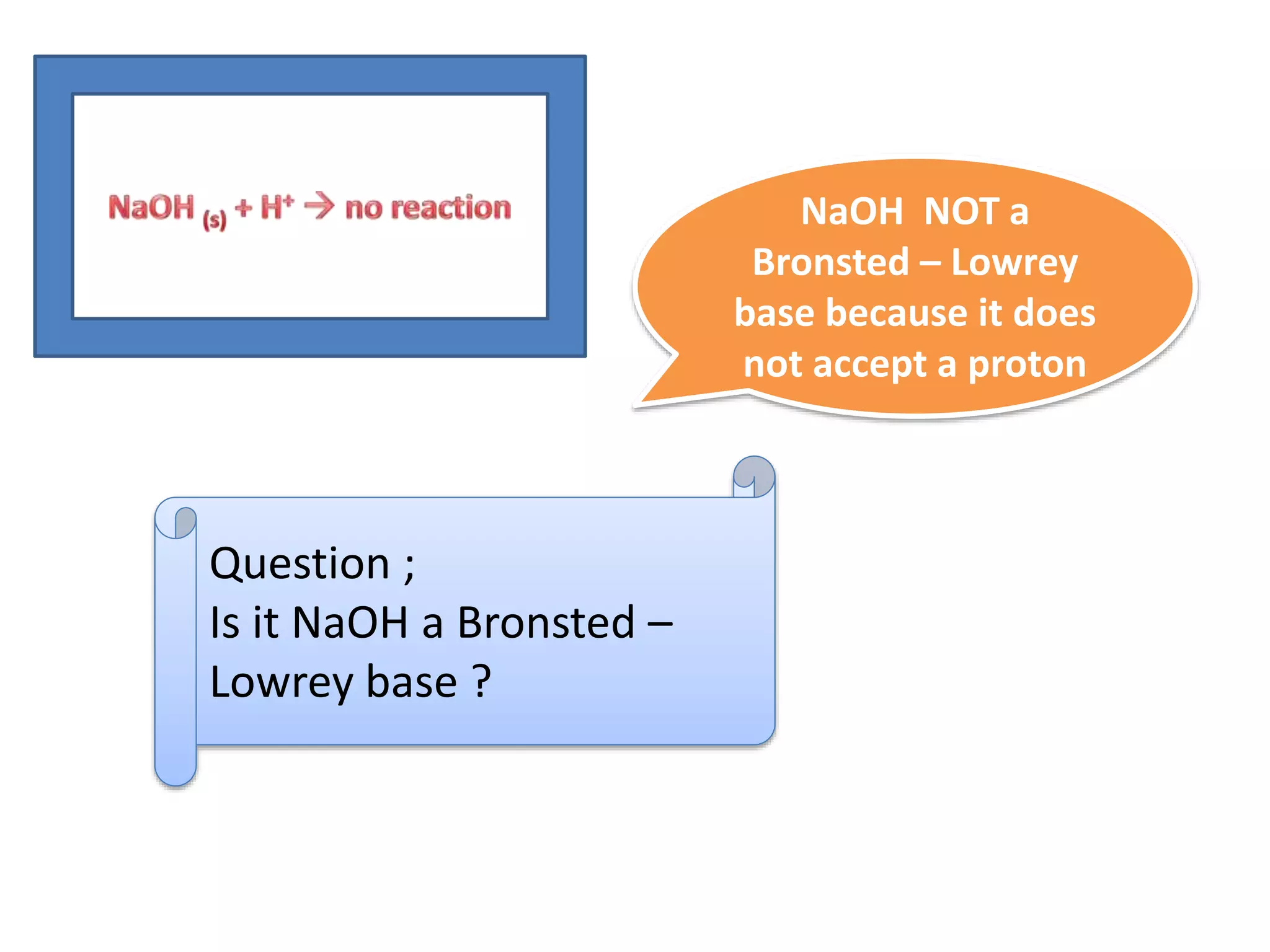
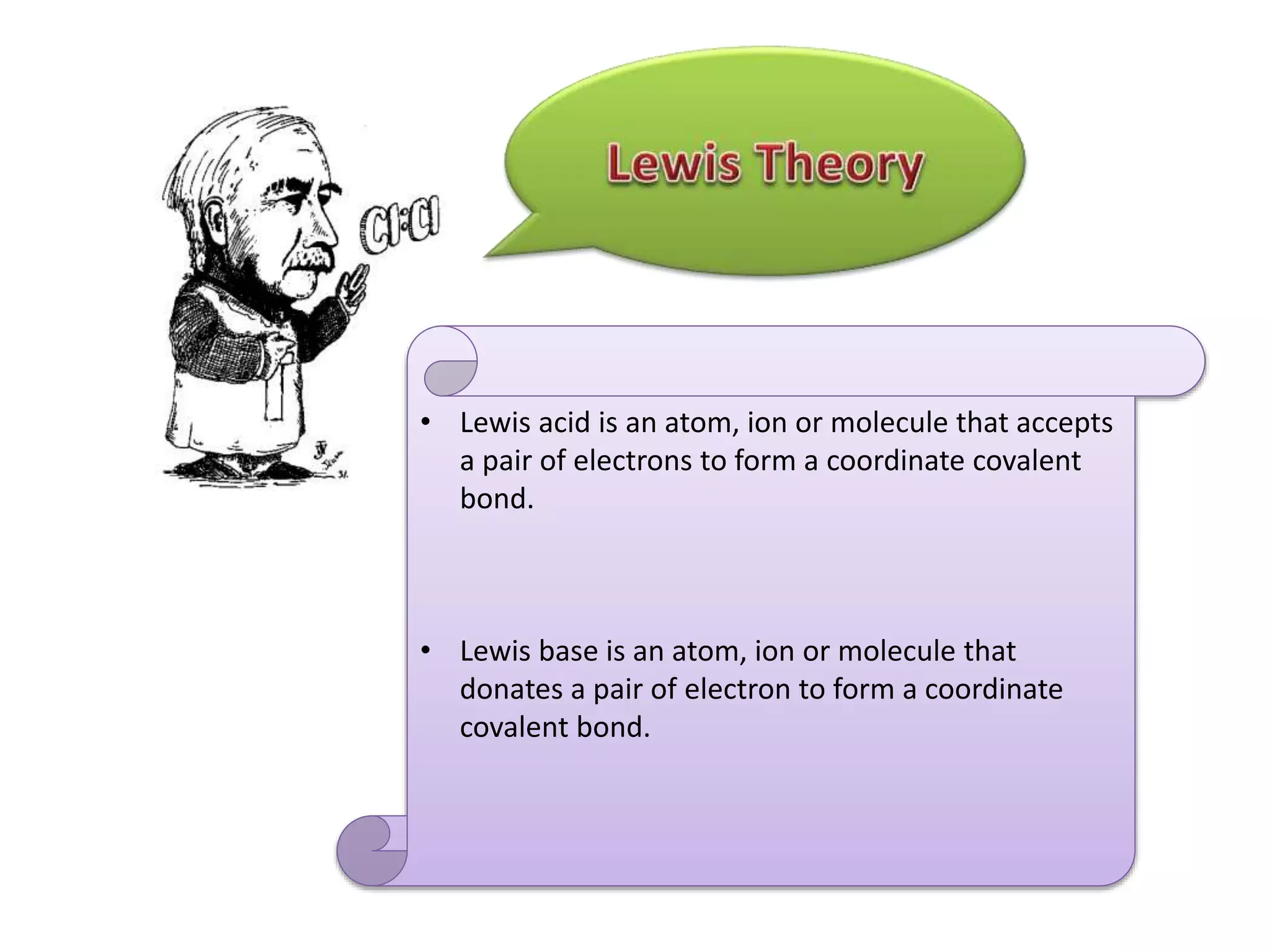
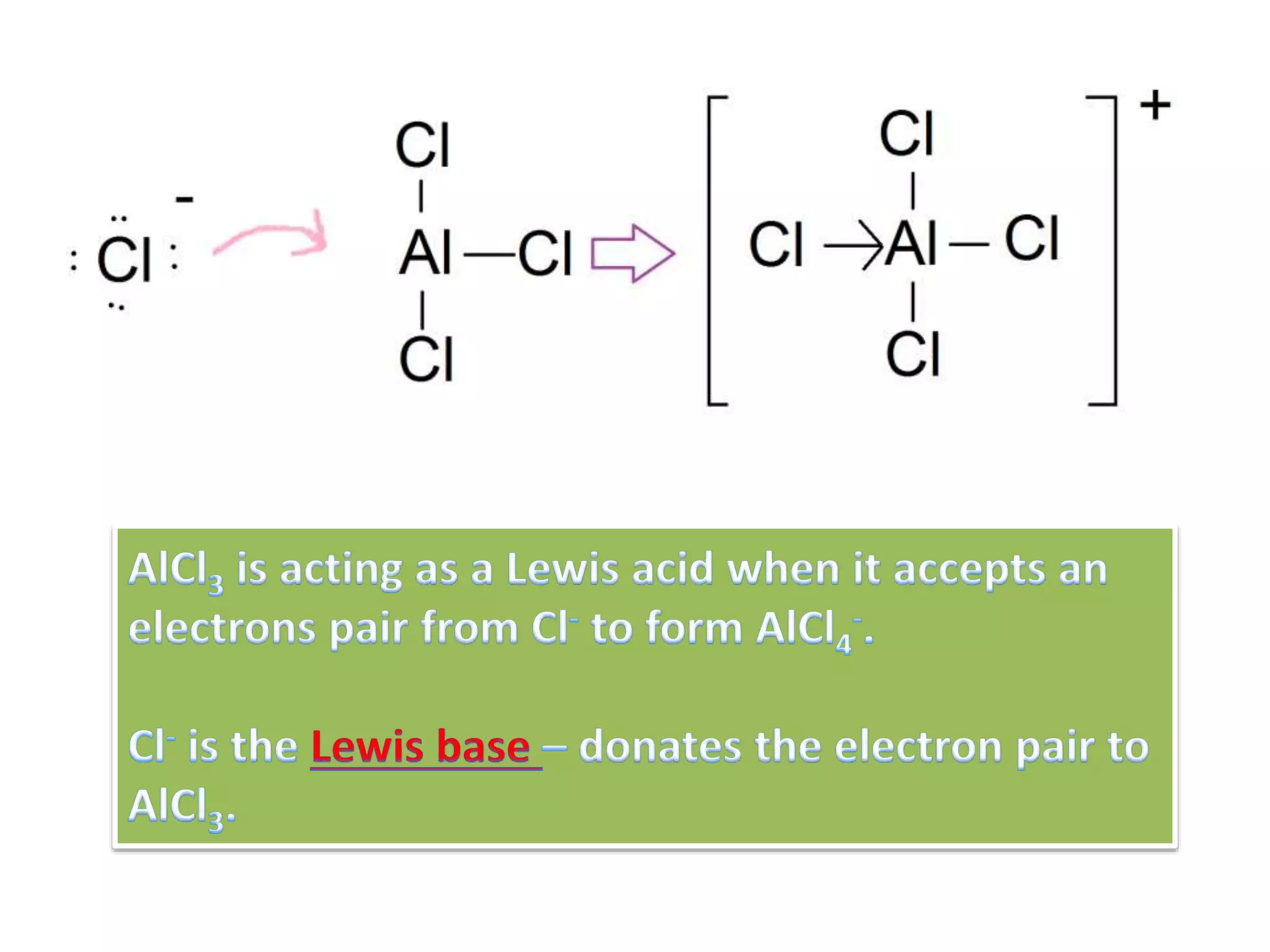
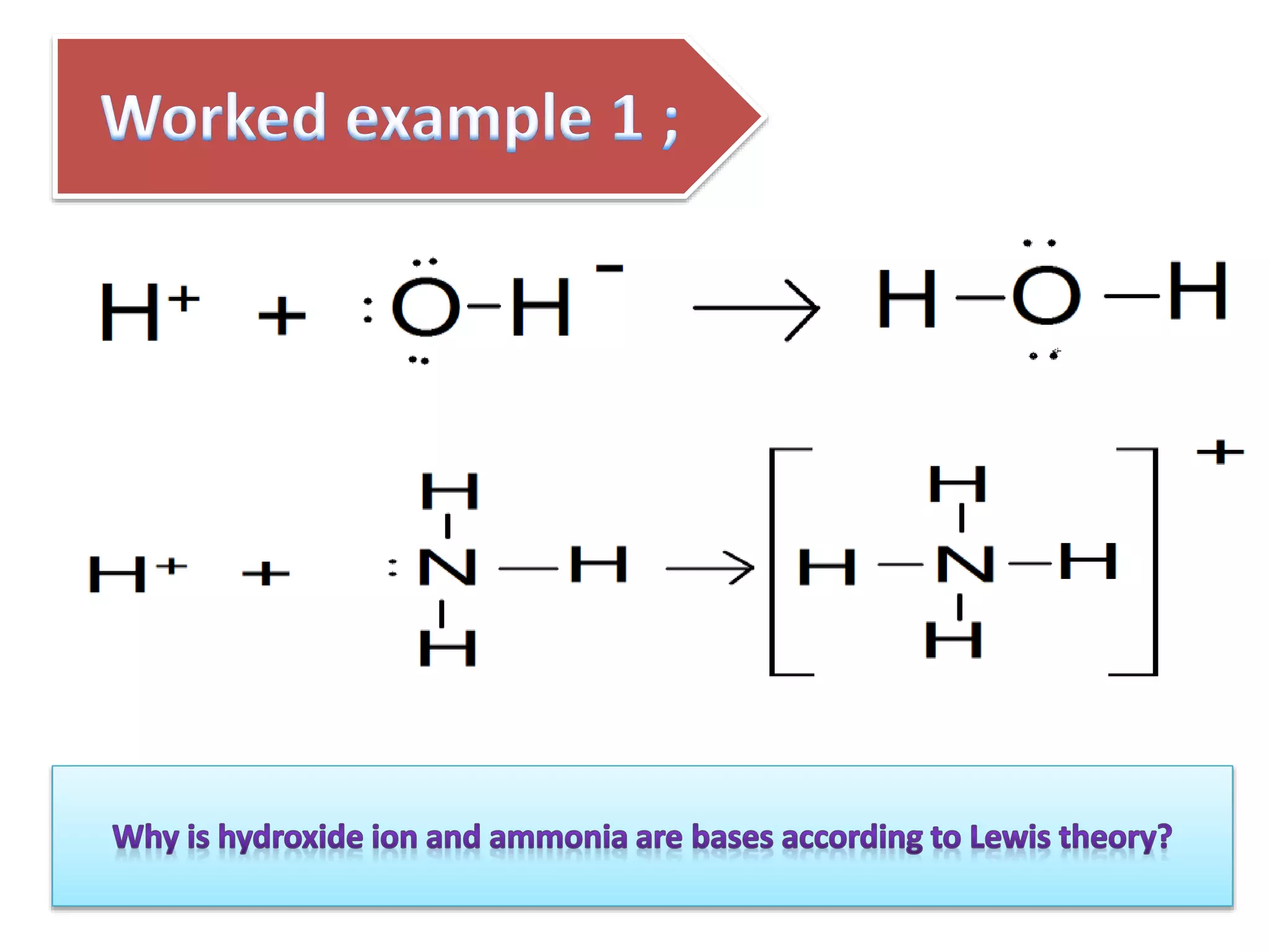
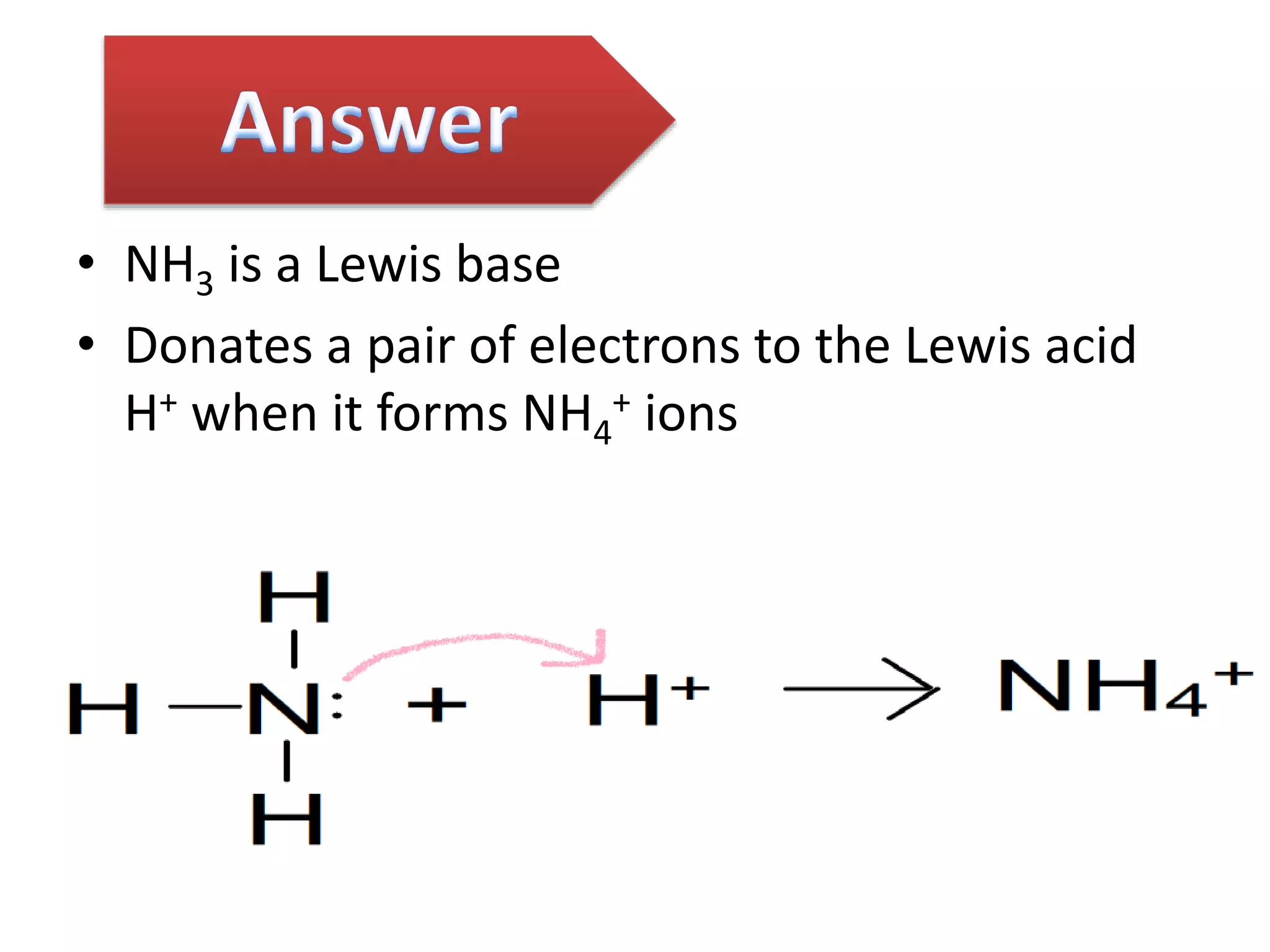
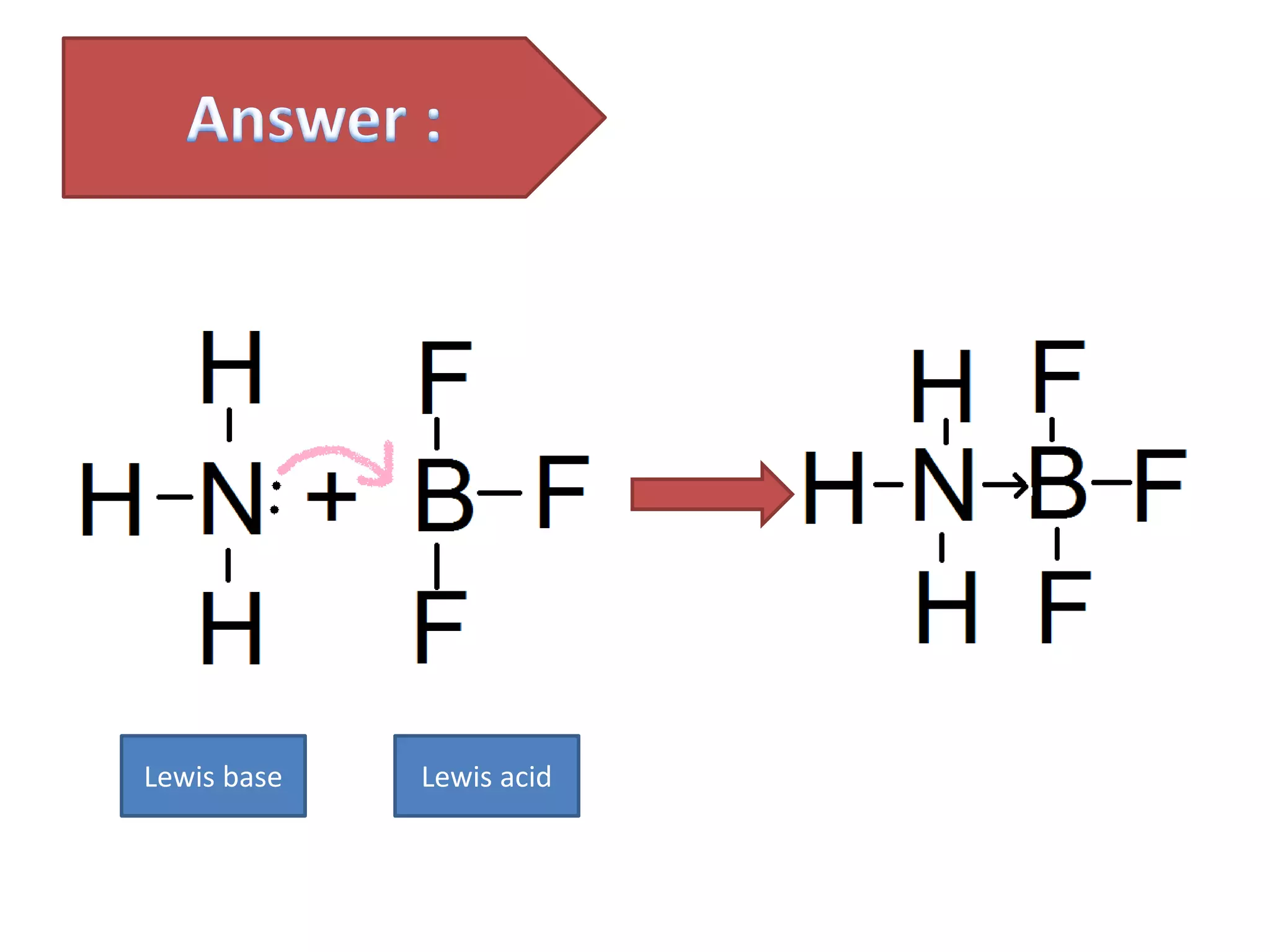
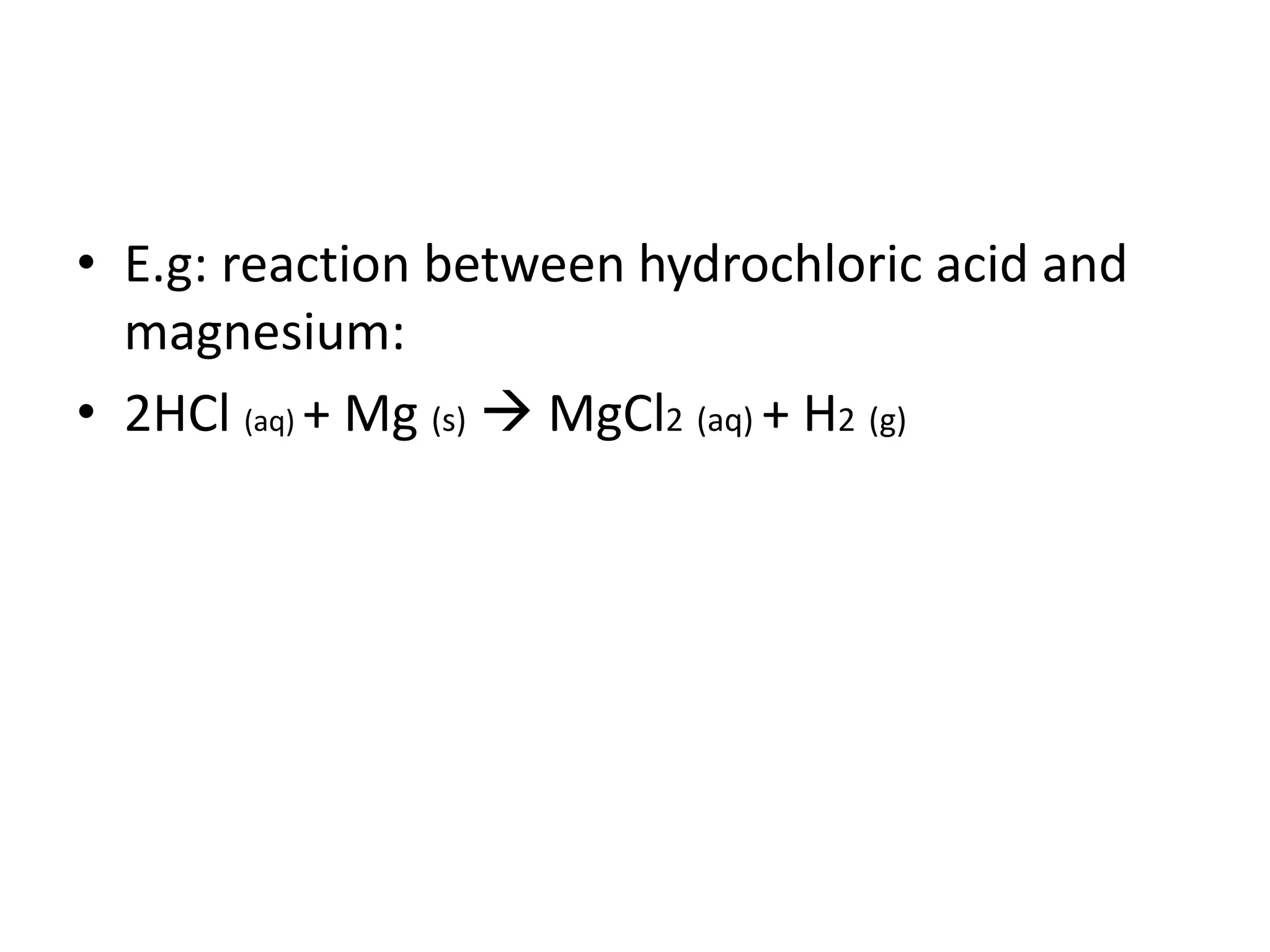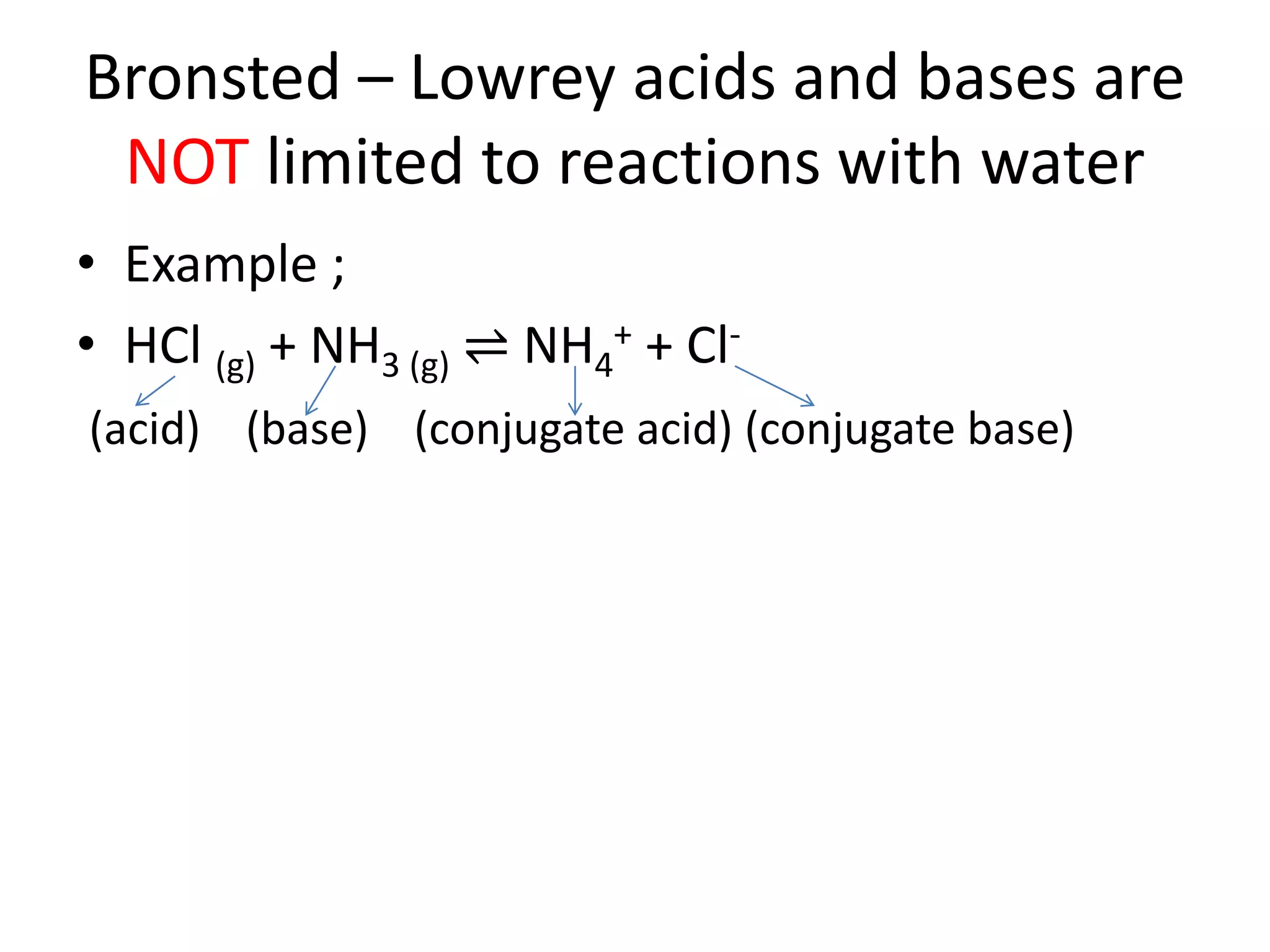This document discusses the properties and reactions of acids and bases. It states that acids have a sour taste and cause color changes in indicators like litmus. Acids react with metals to produce hydrogen gas and with carbonates to produce carbon dioxide gas. Bases have a bitter taste and feel slippery. Strong acids and bases fully dissociate in water while weak acids and bases only partially dissociate. The Bronsted-Lowry theory defines acids as proton donors and bases as proton acceptors. Water can act as both an acid and a base depending on the reaction.