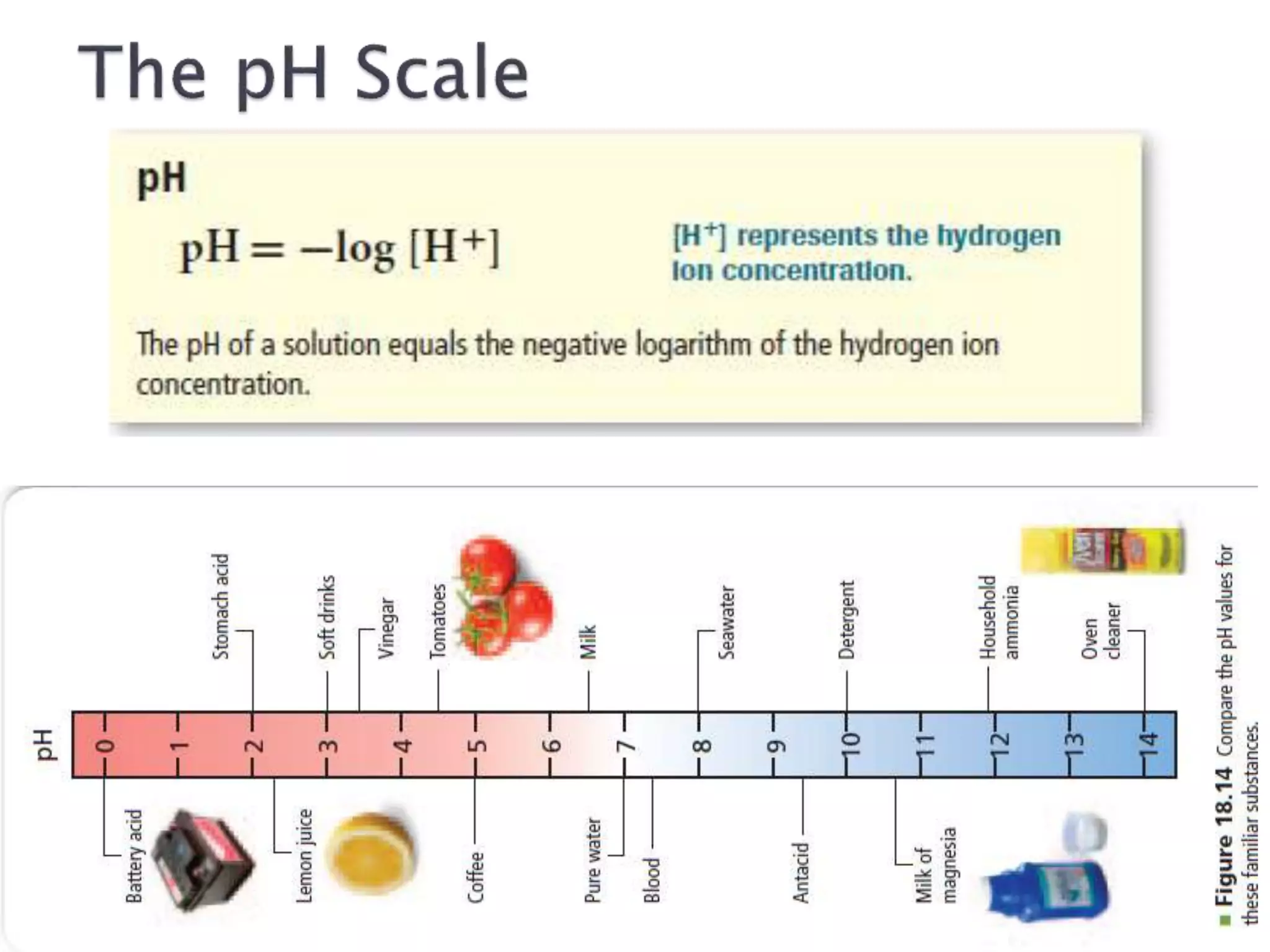
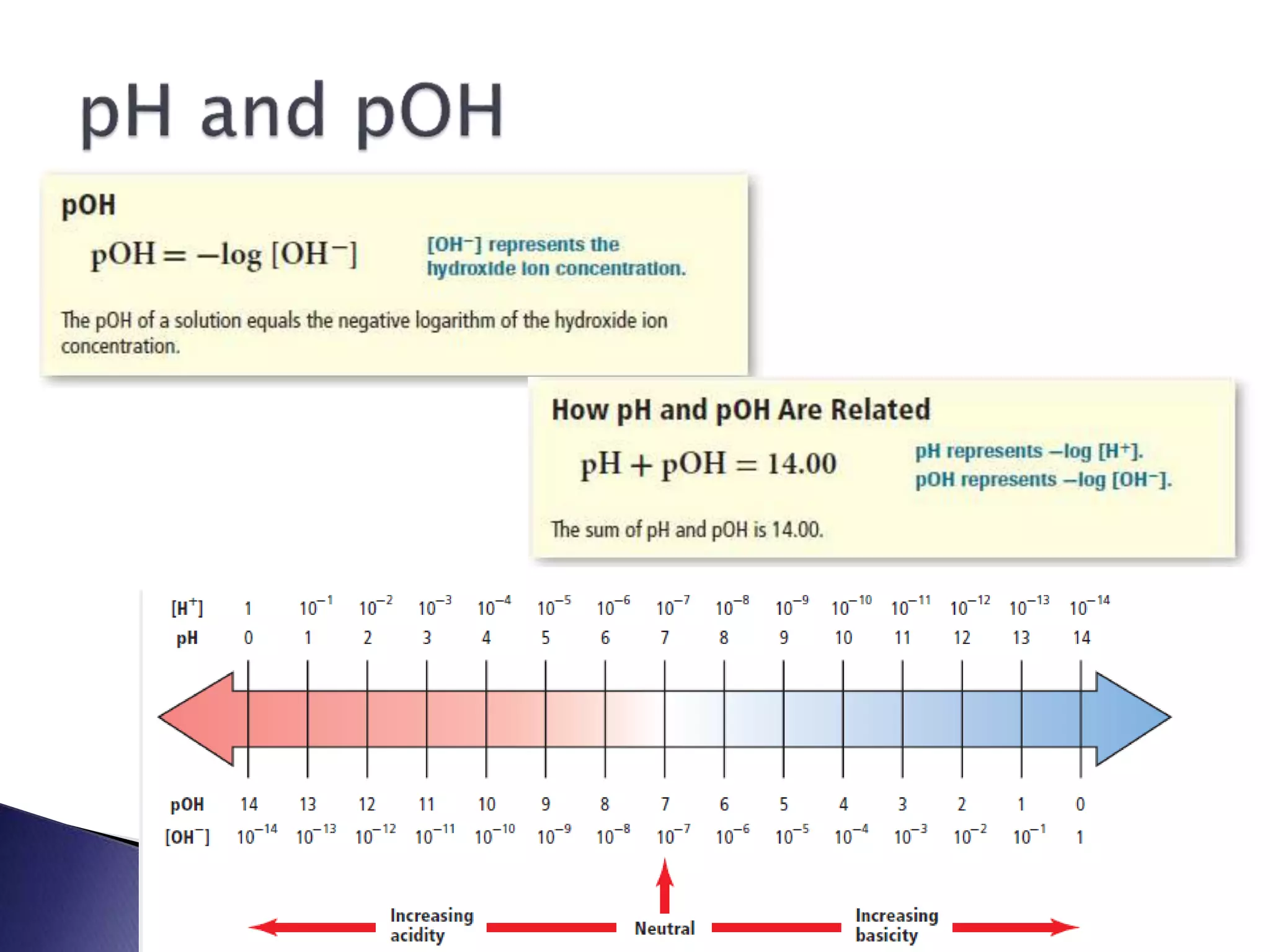
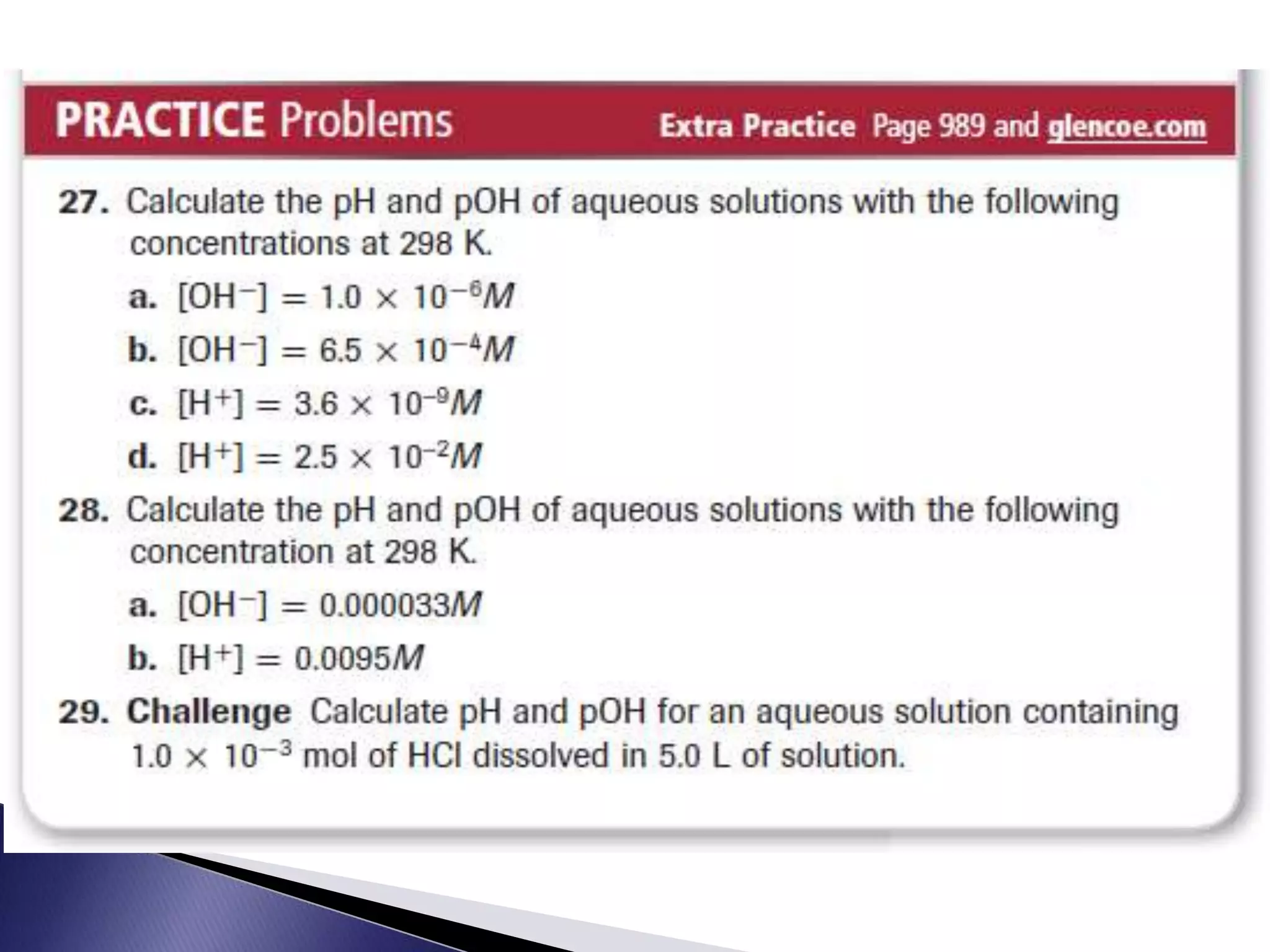
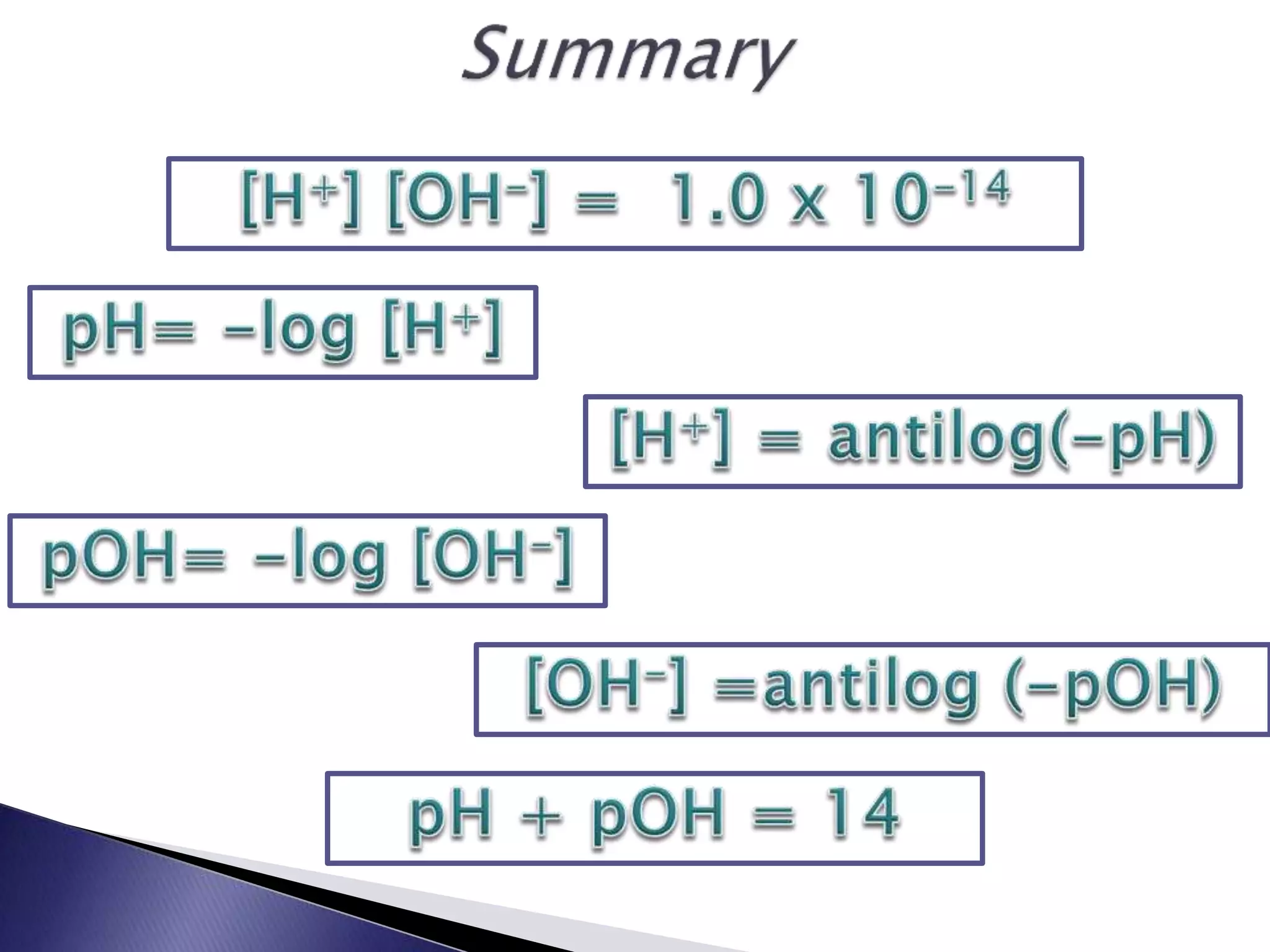
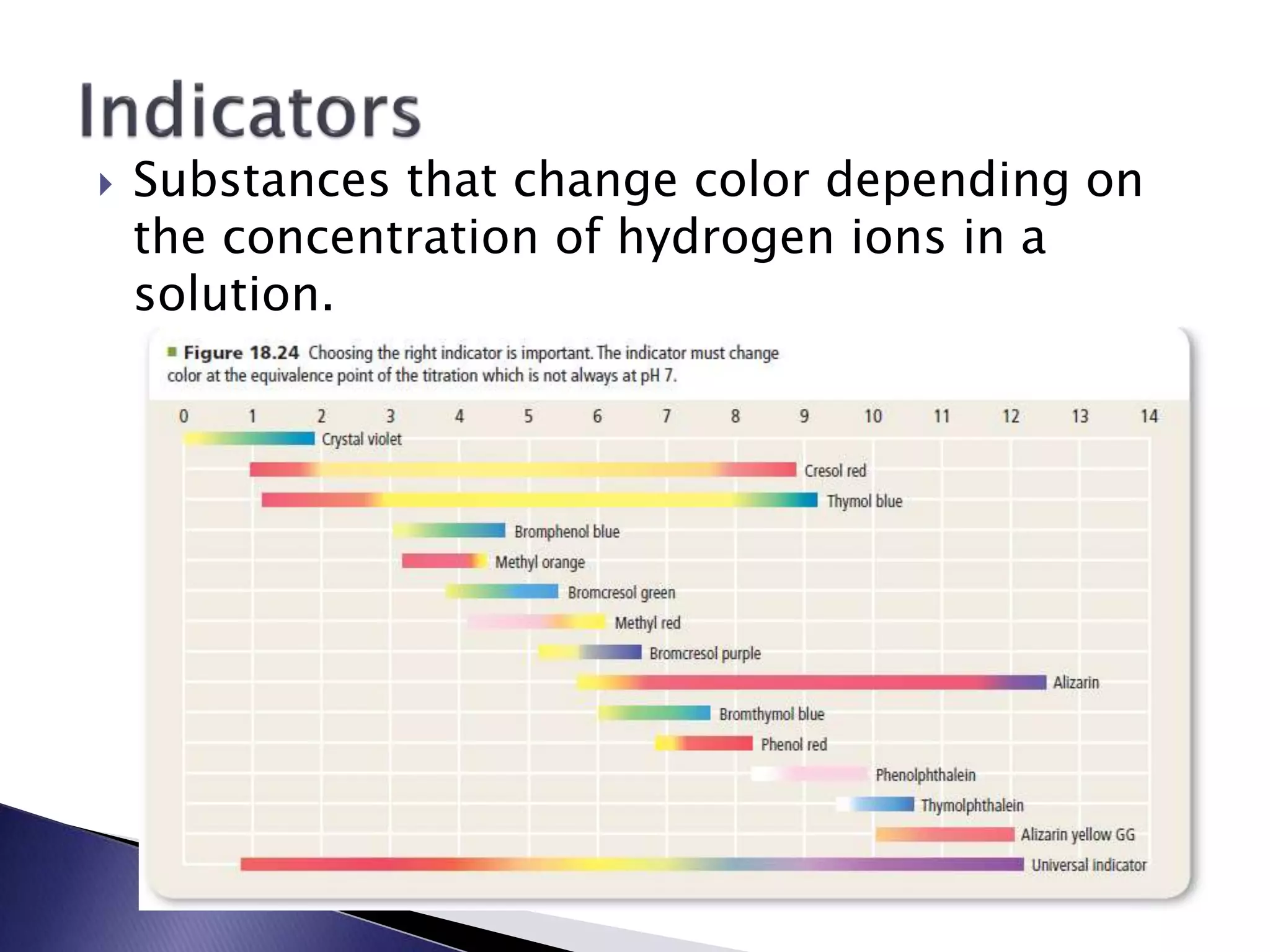
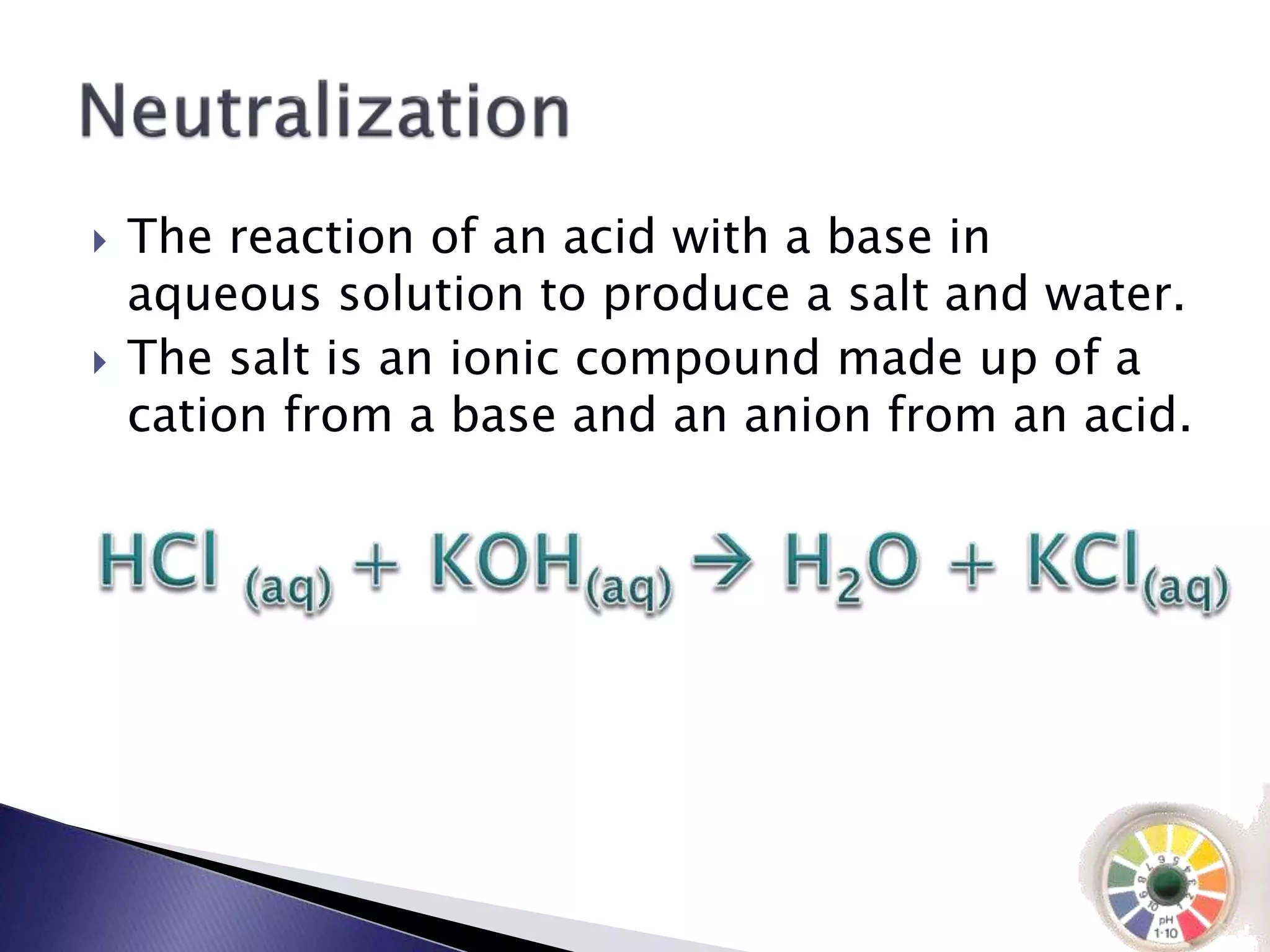
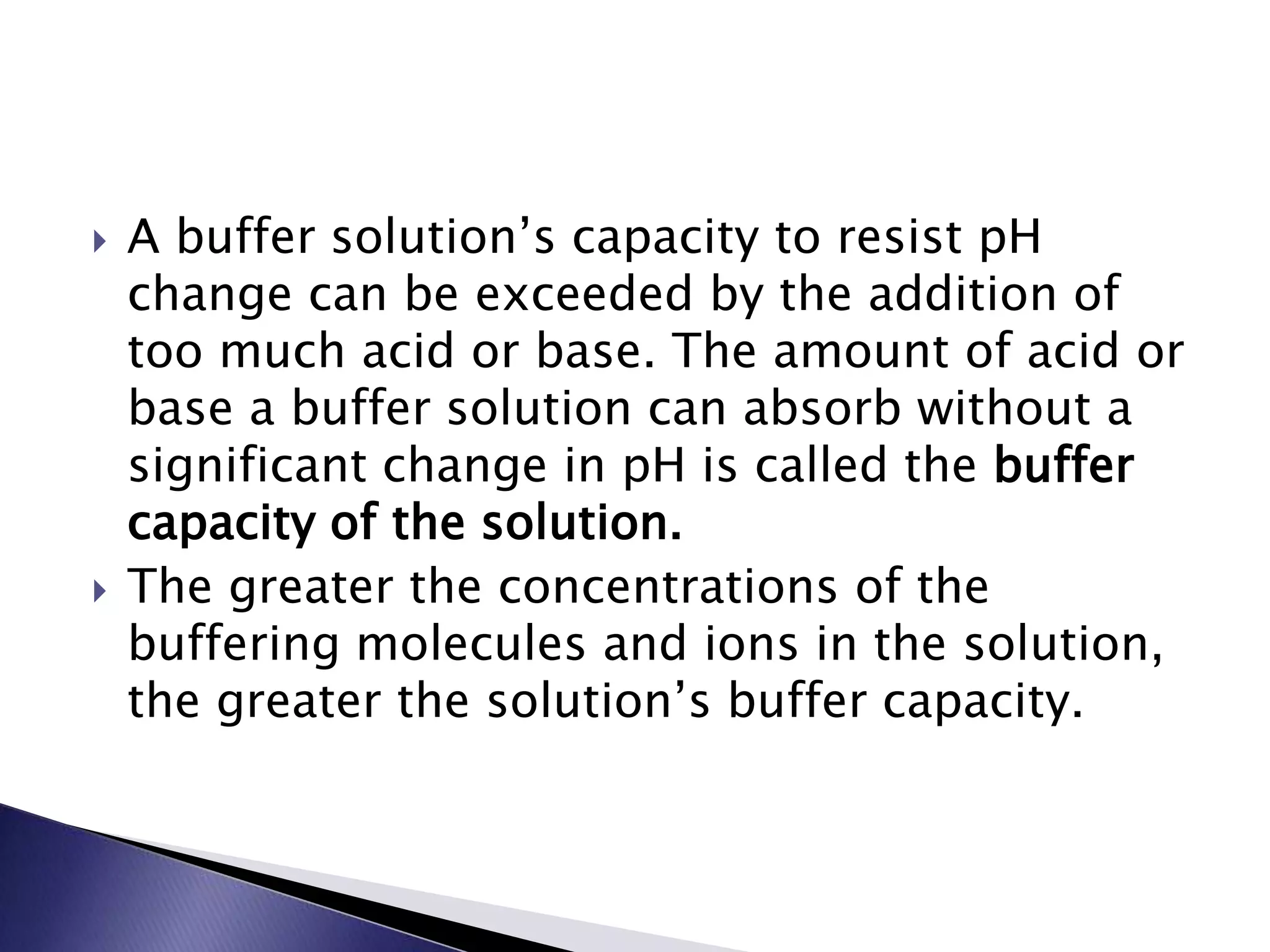
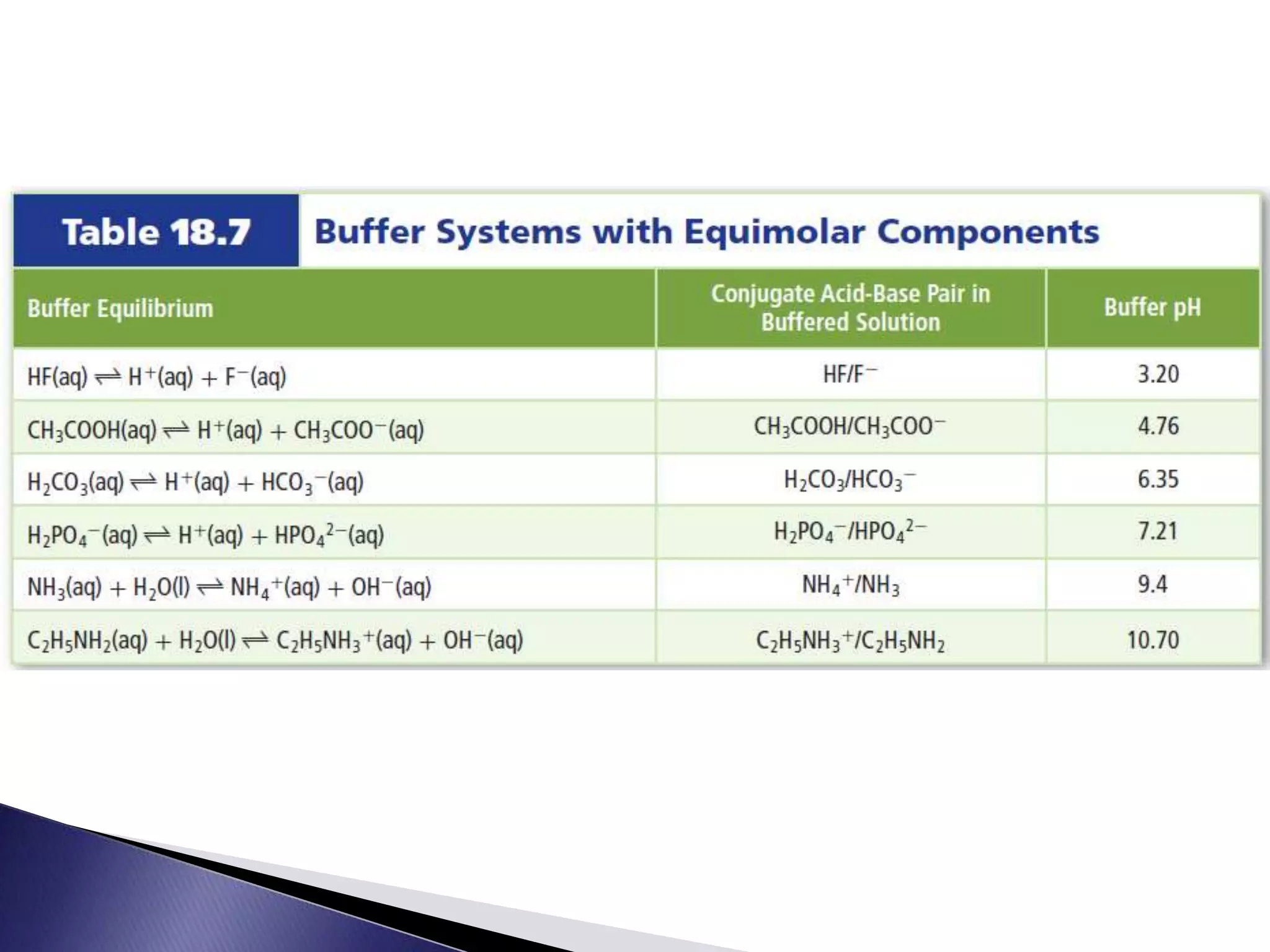
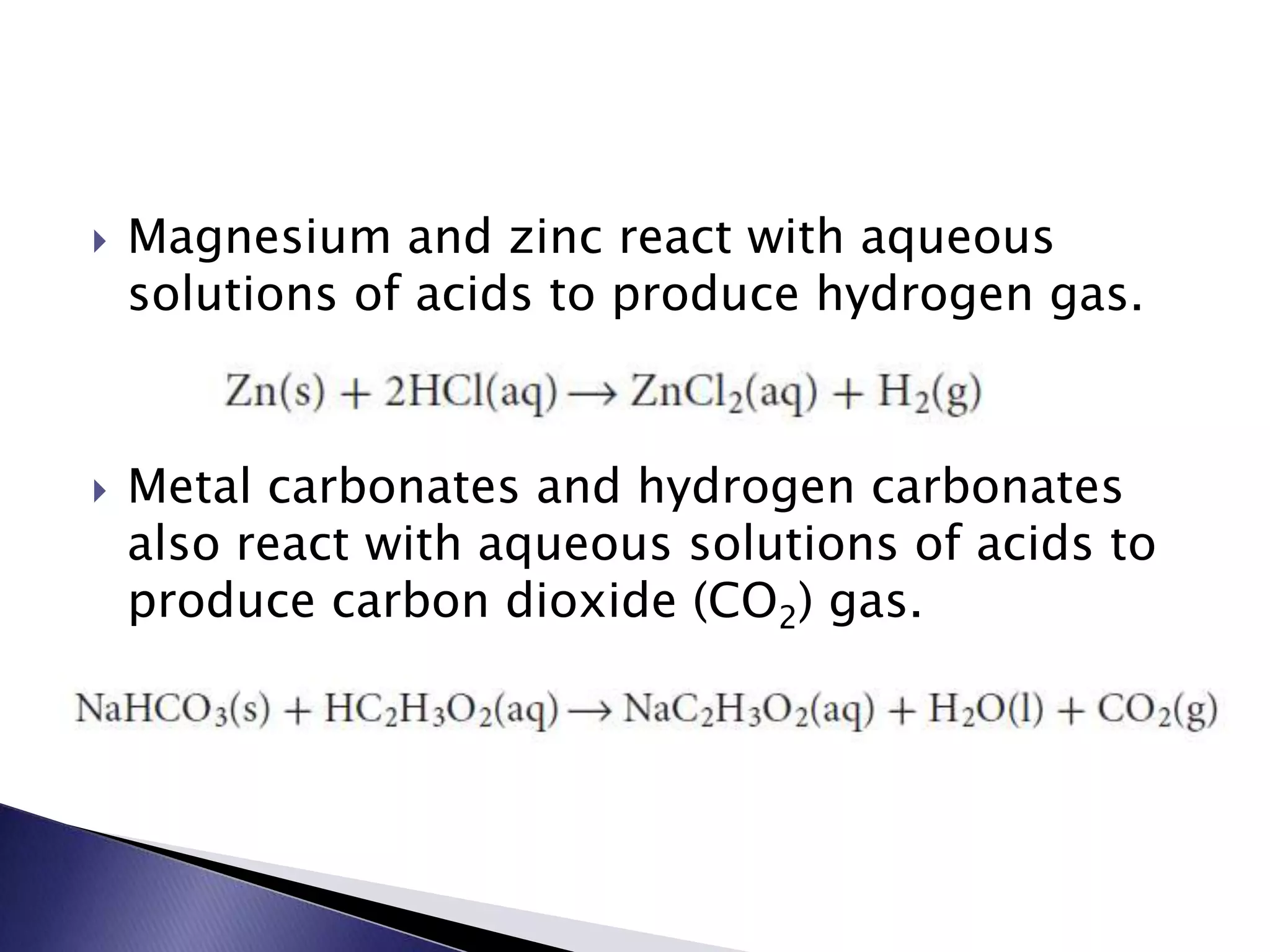
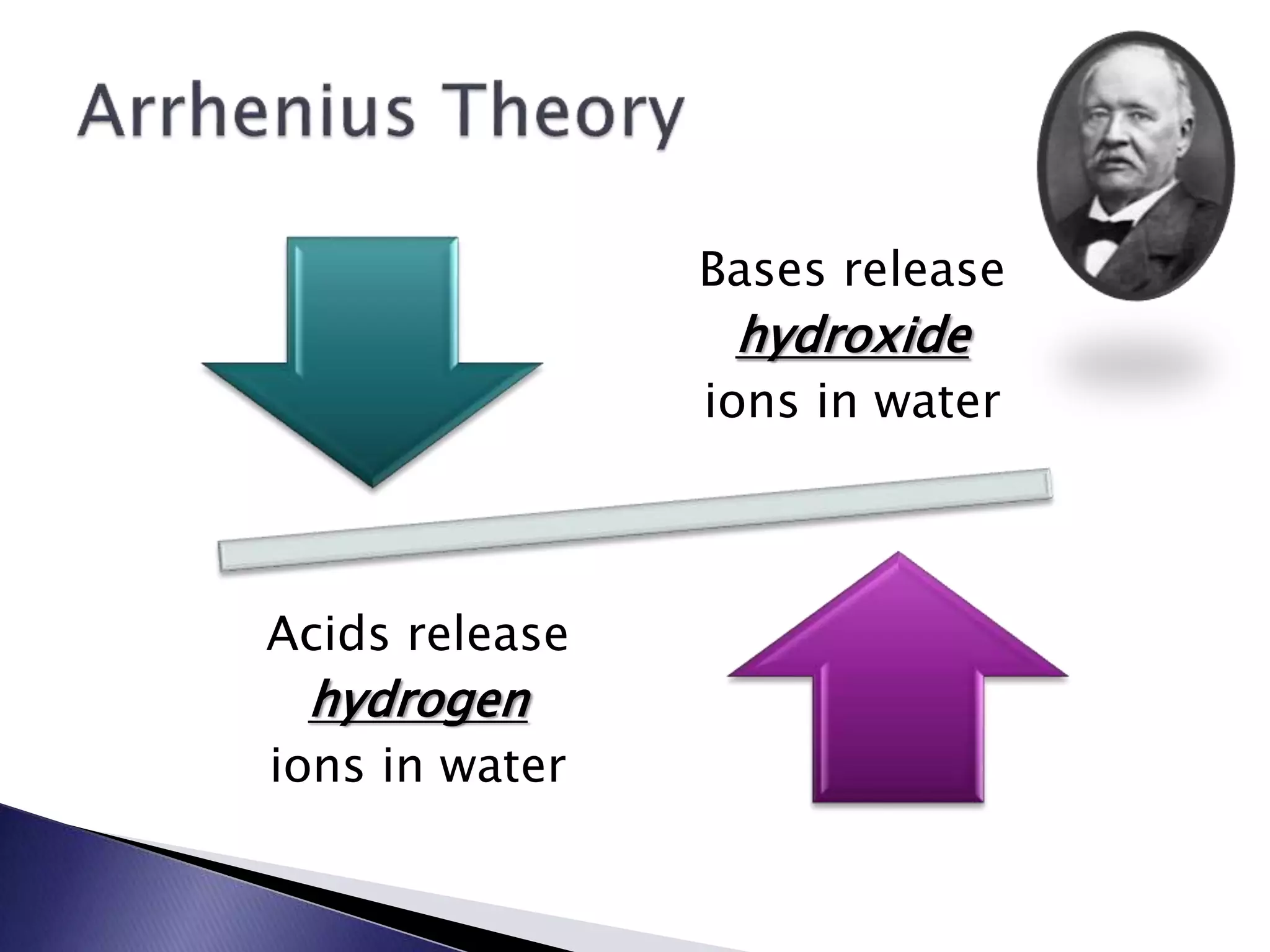
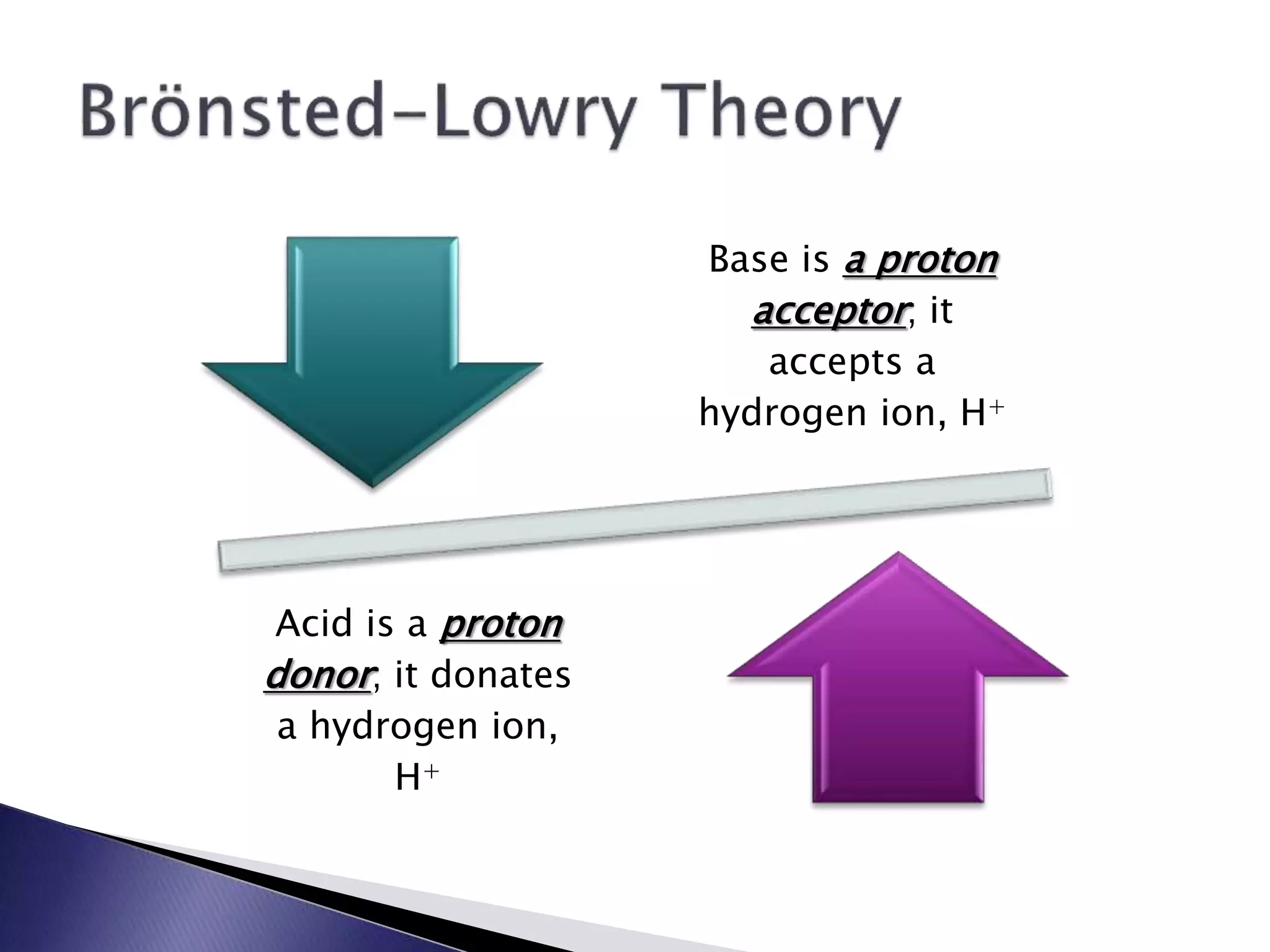
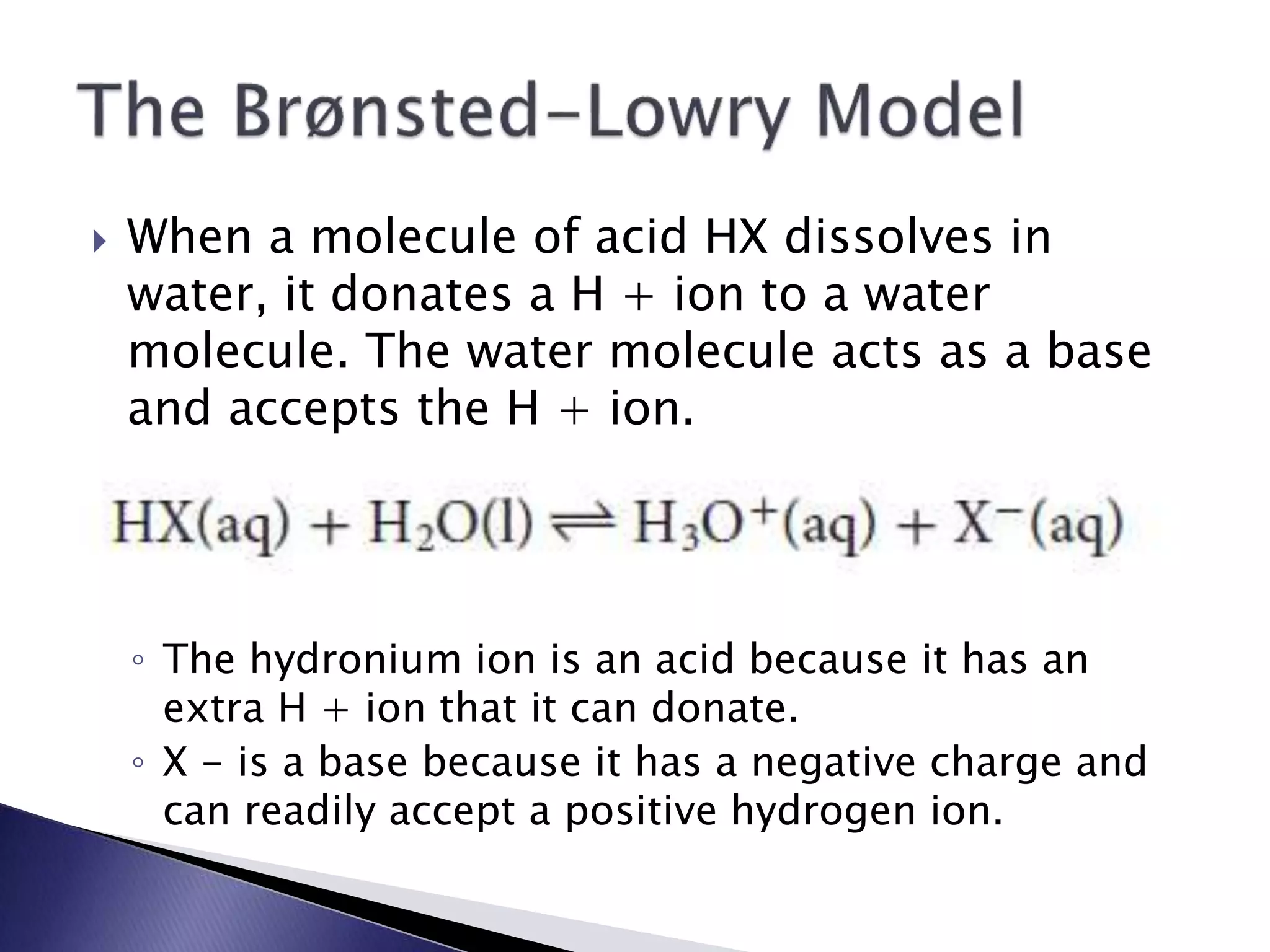
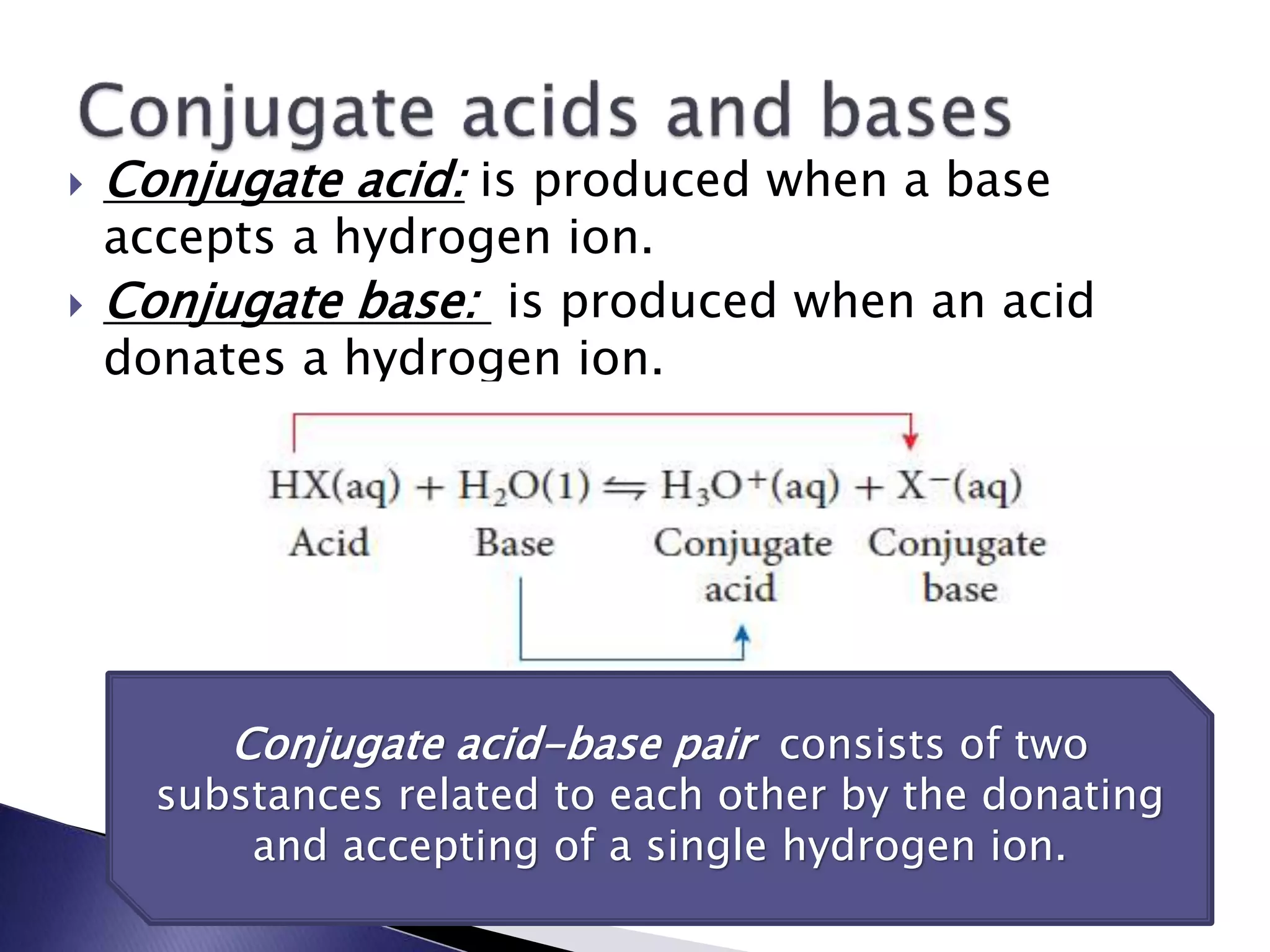
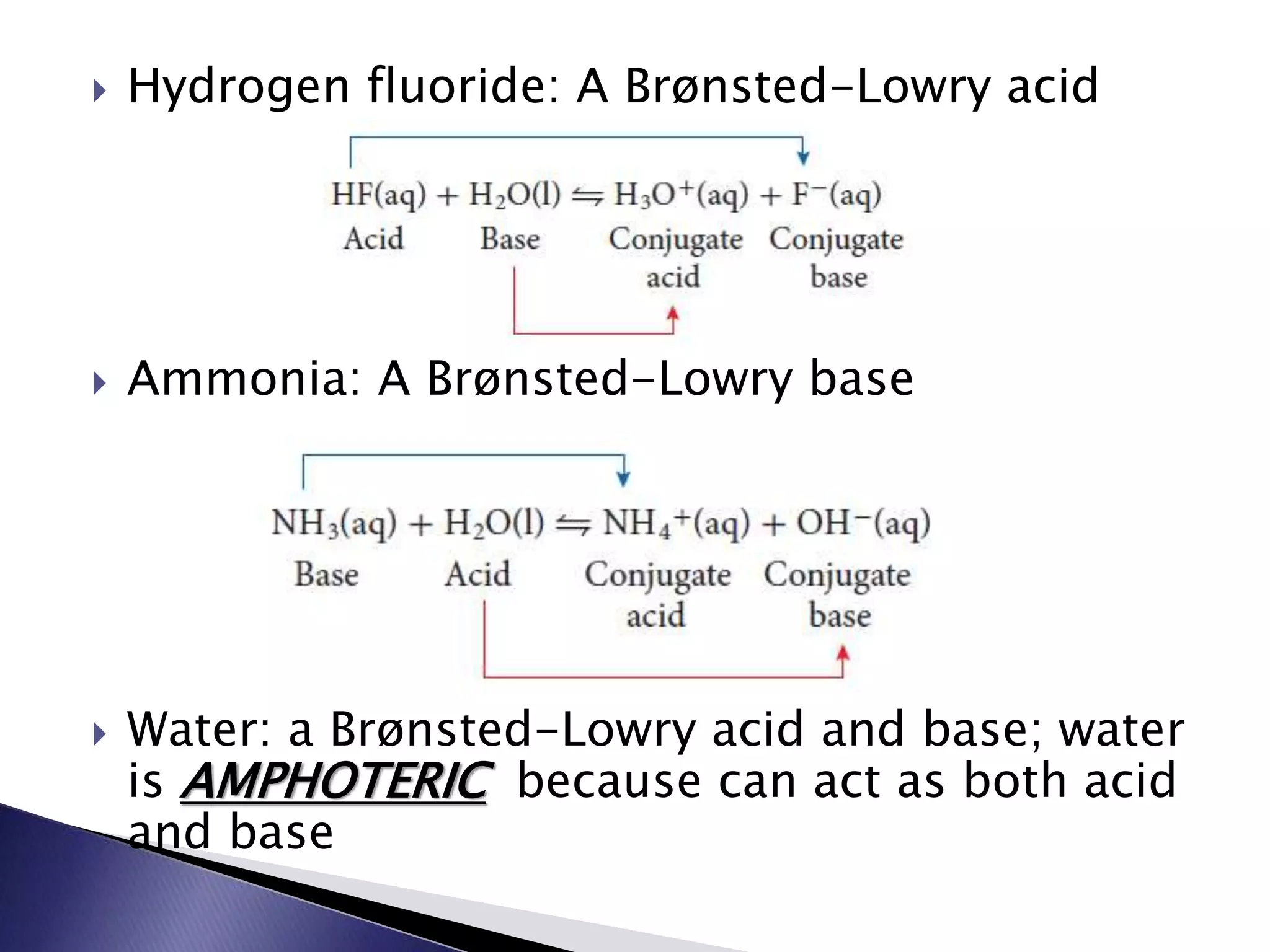
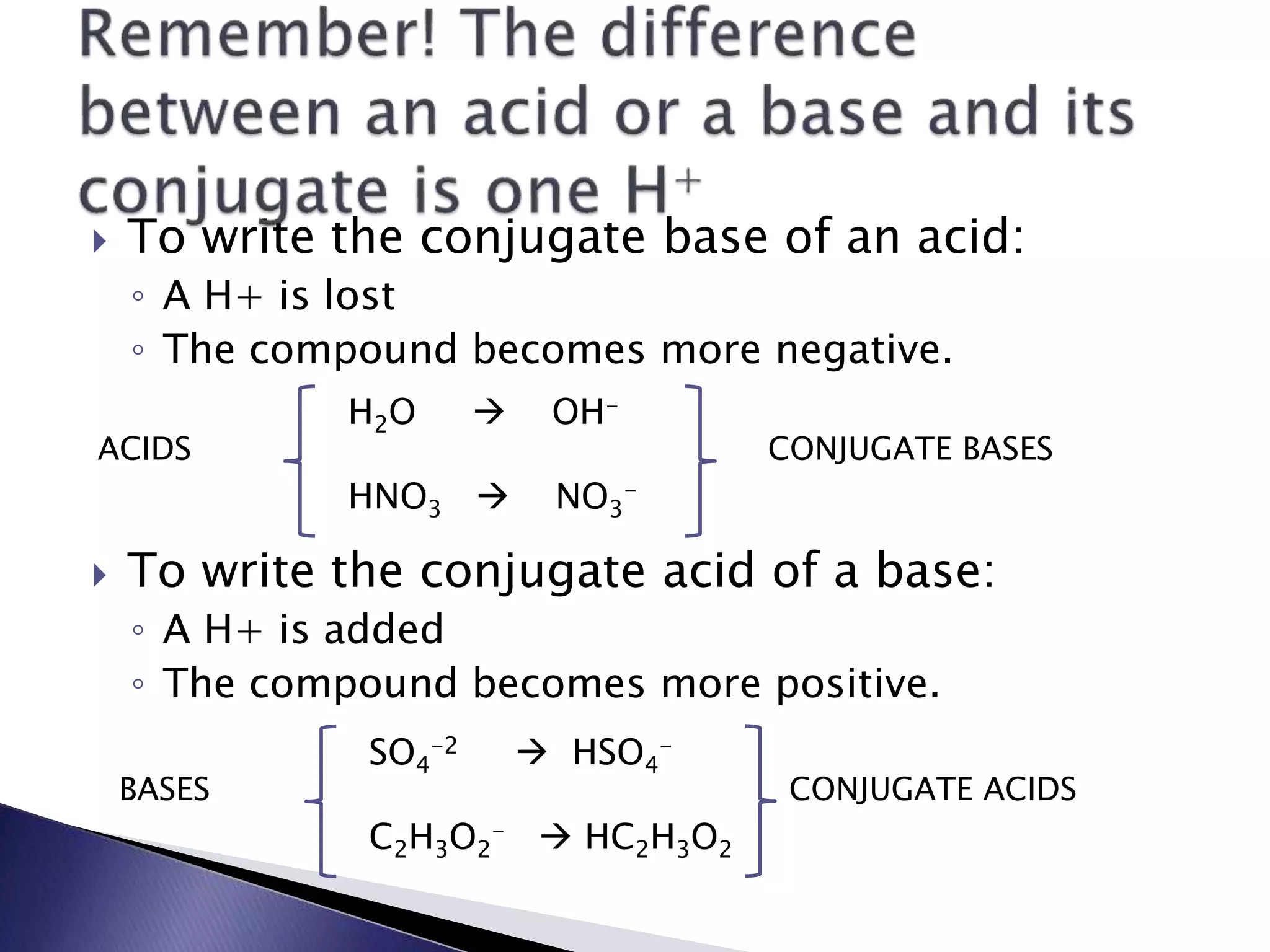
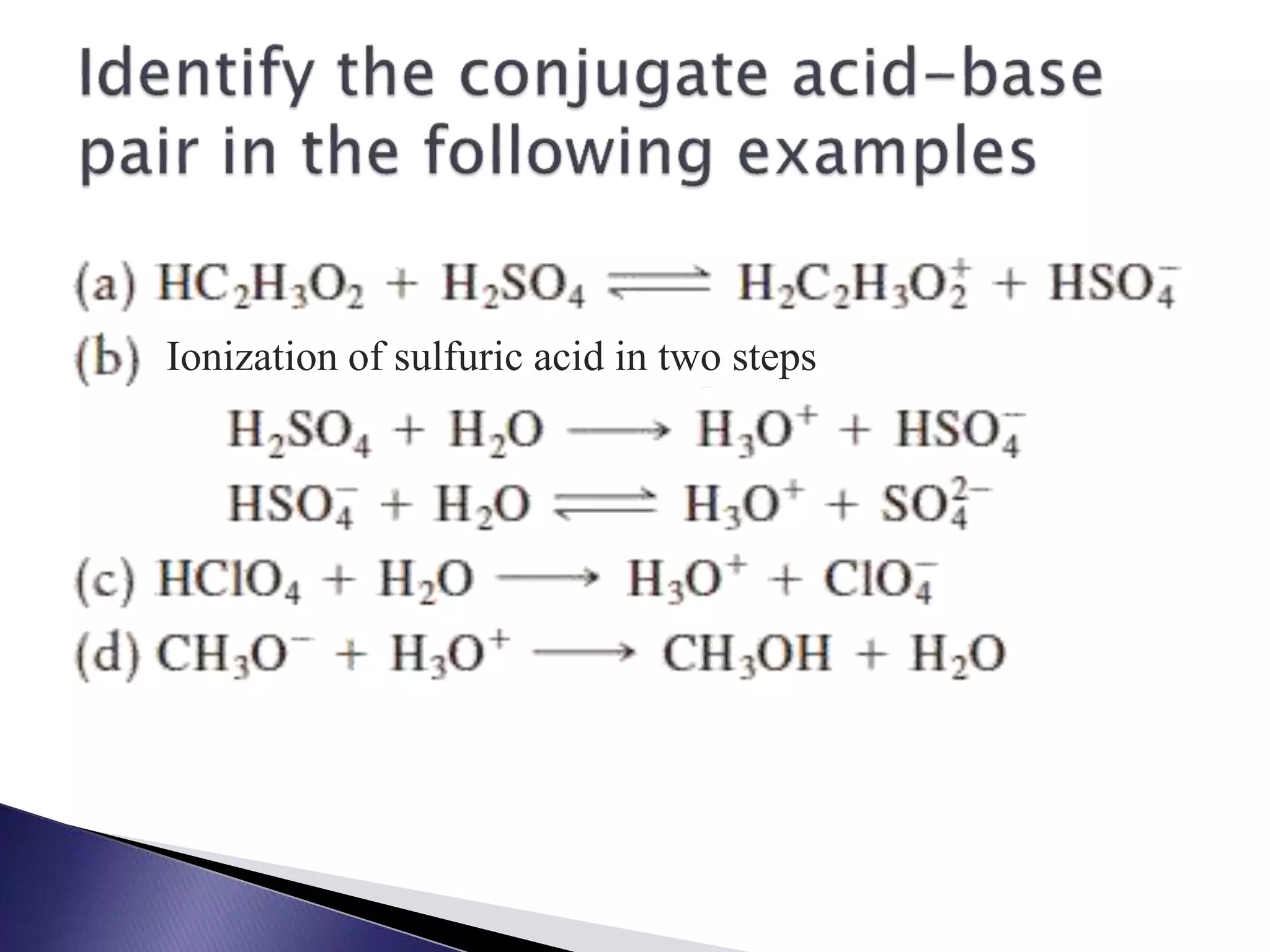
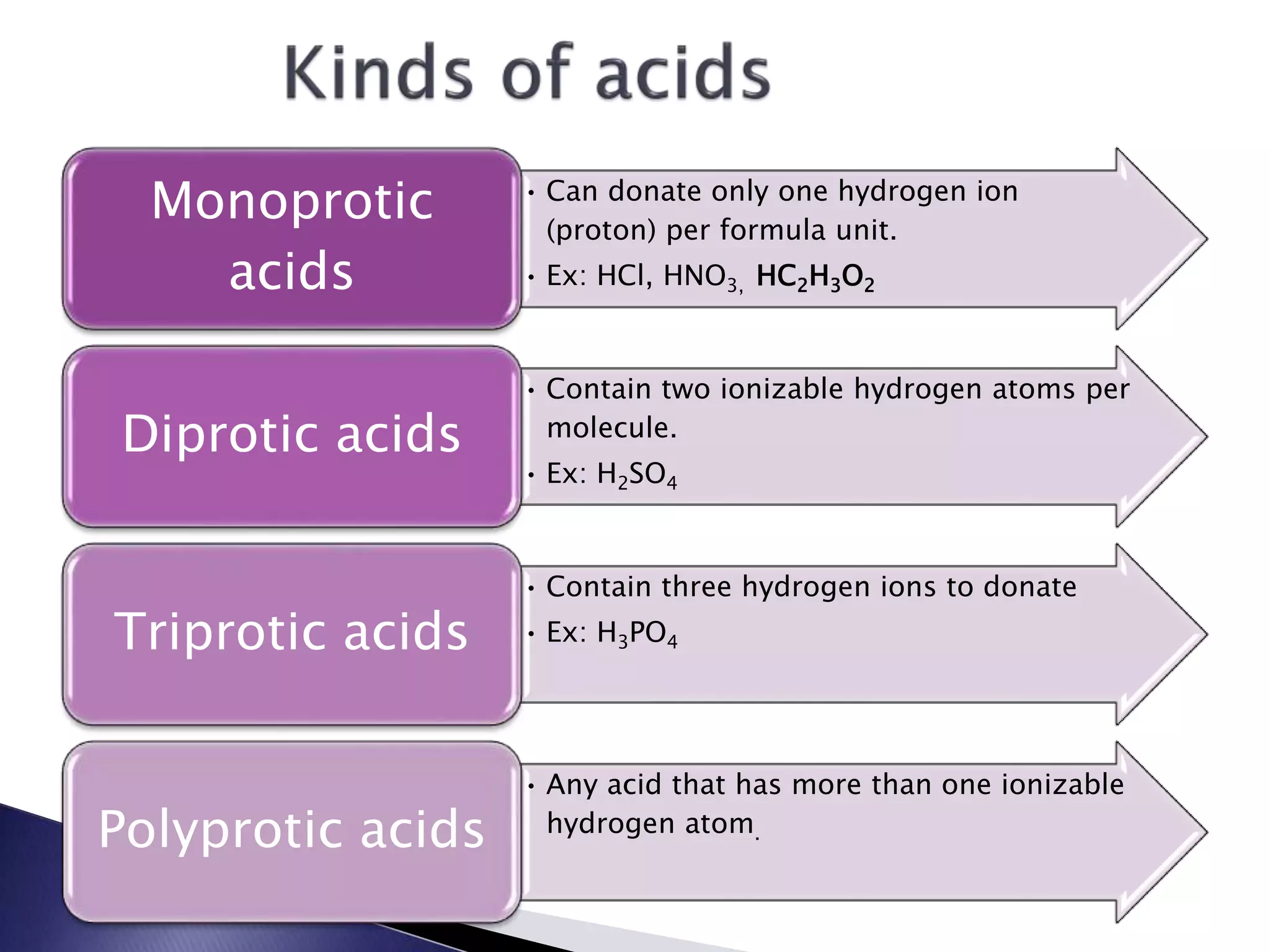
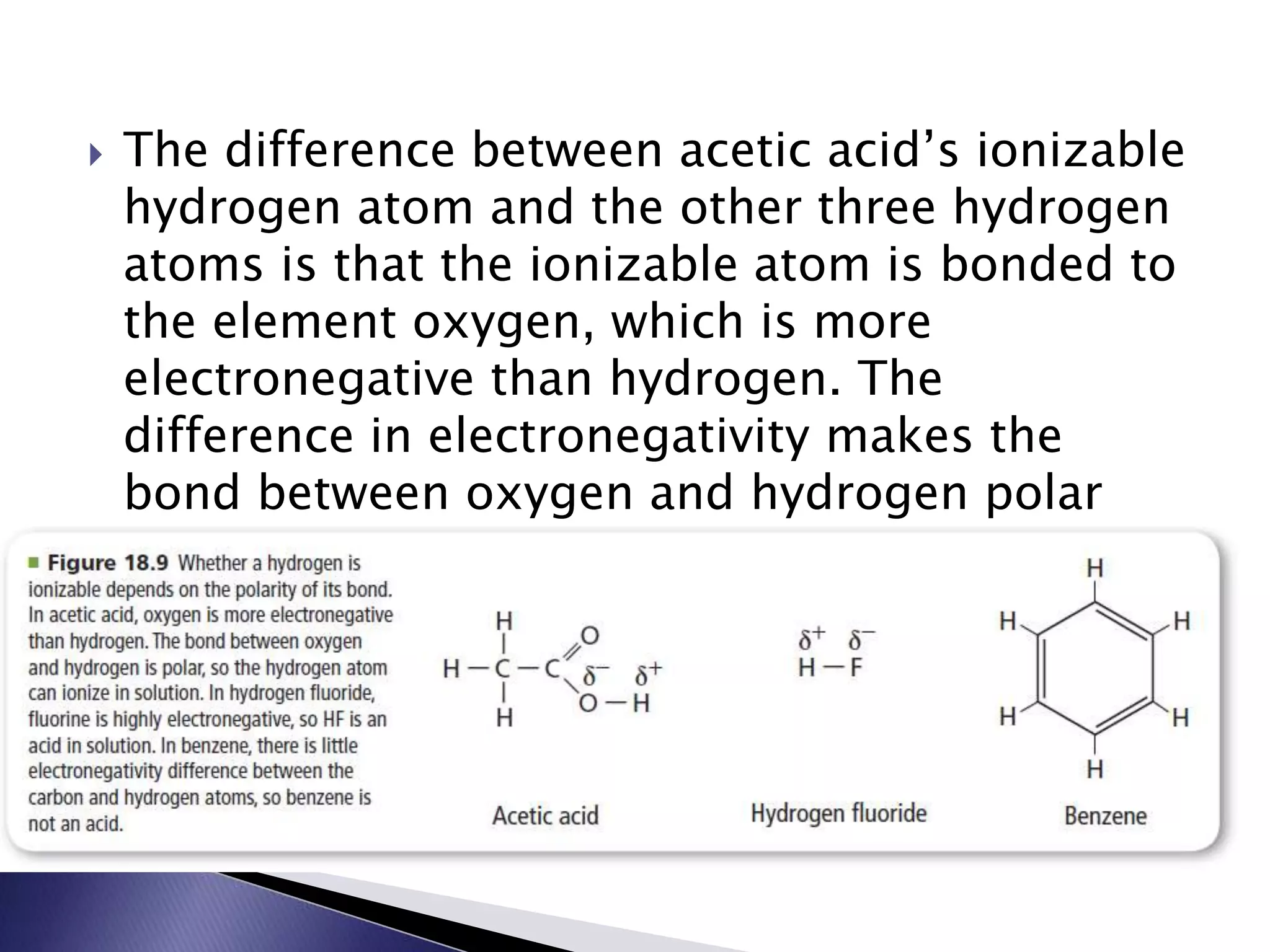
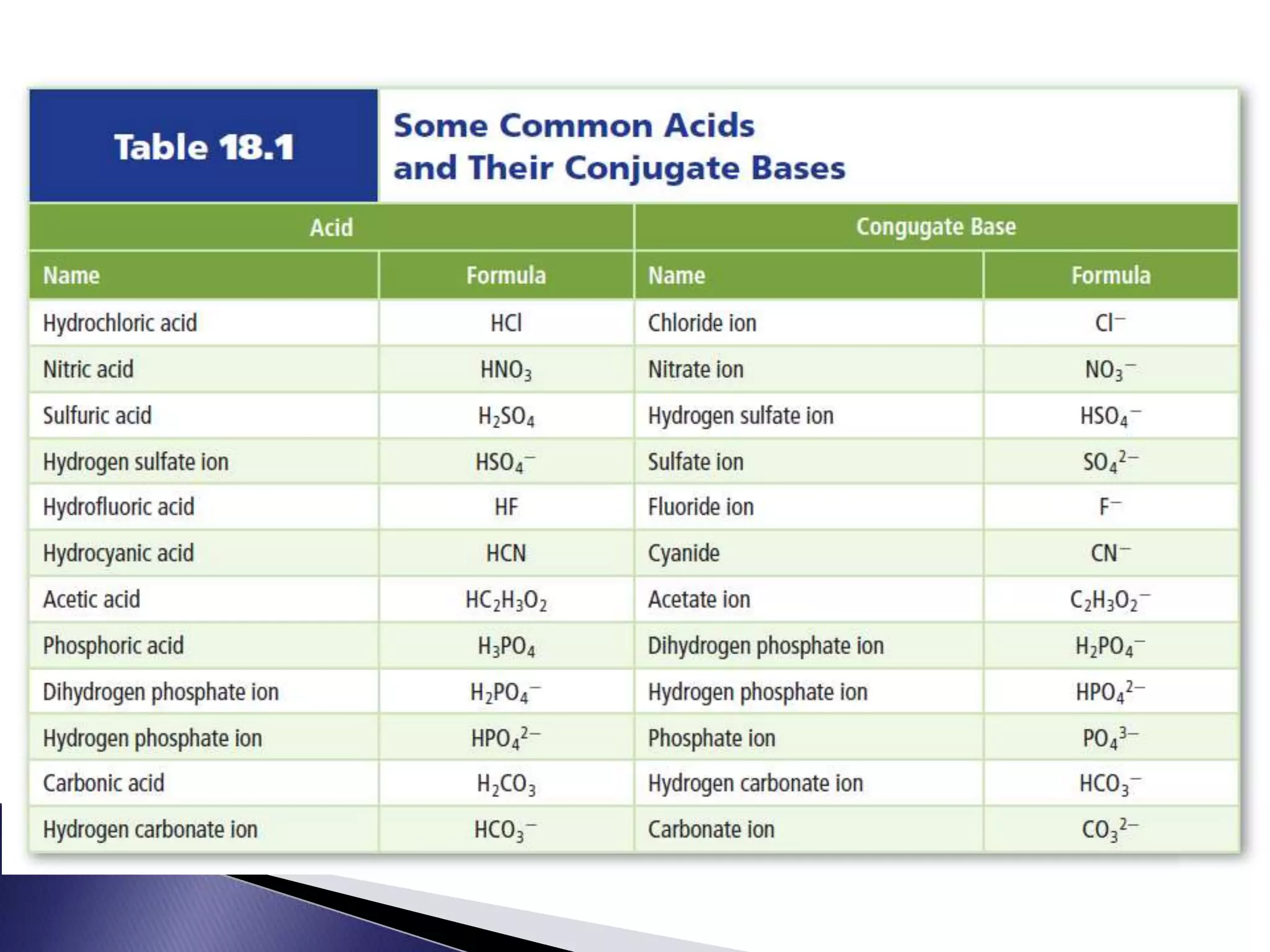
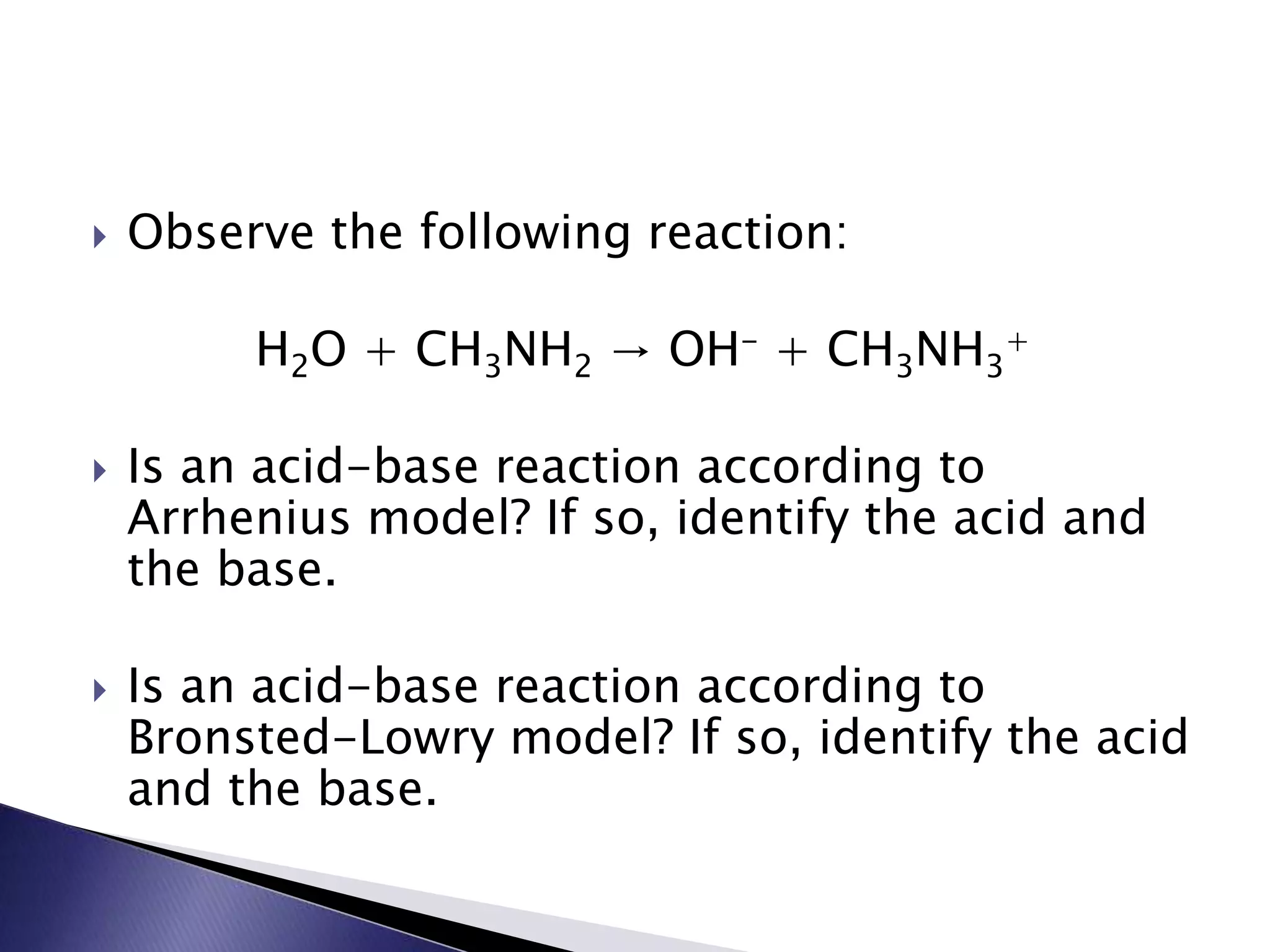
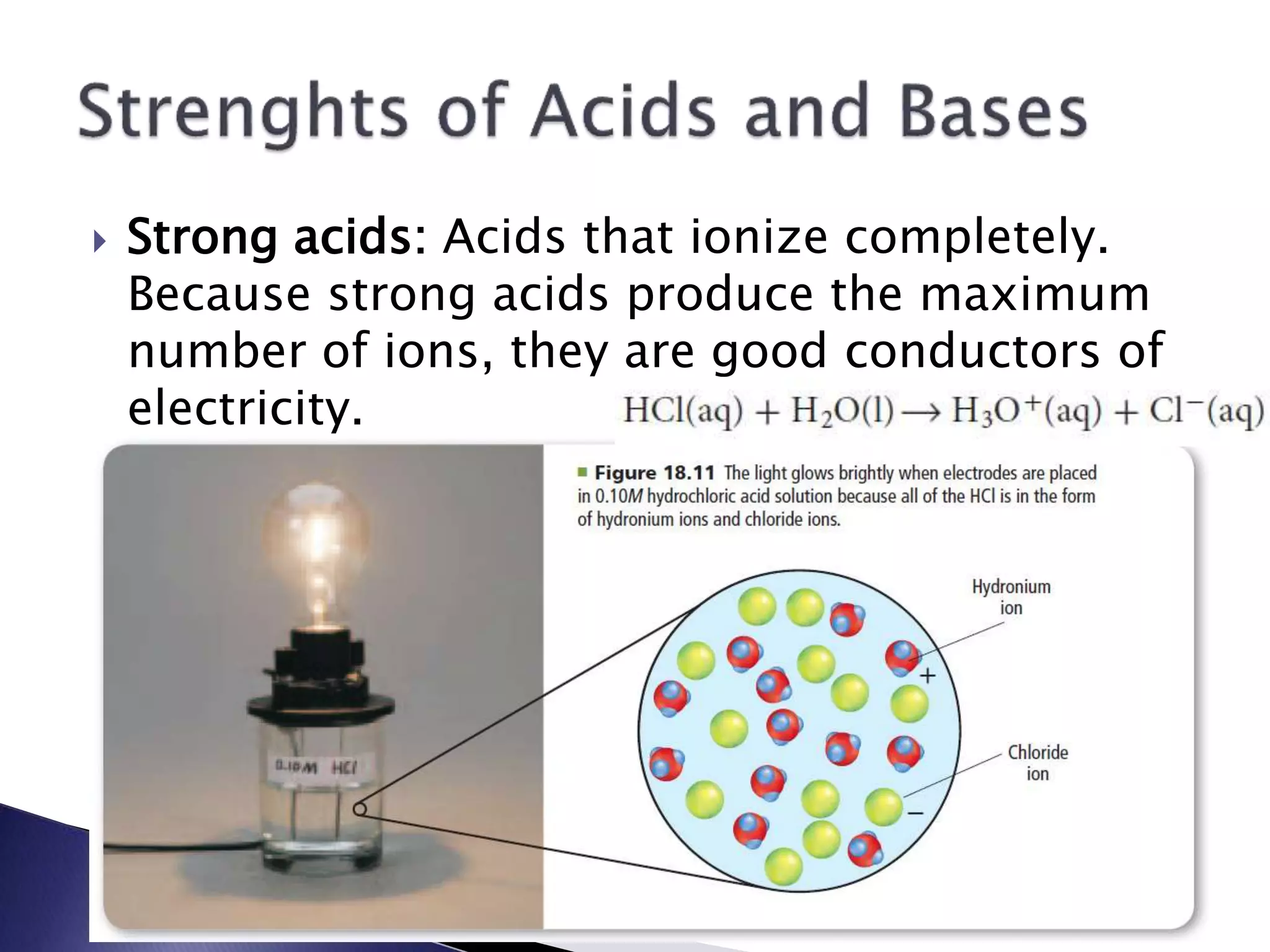
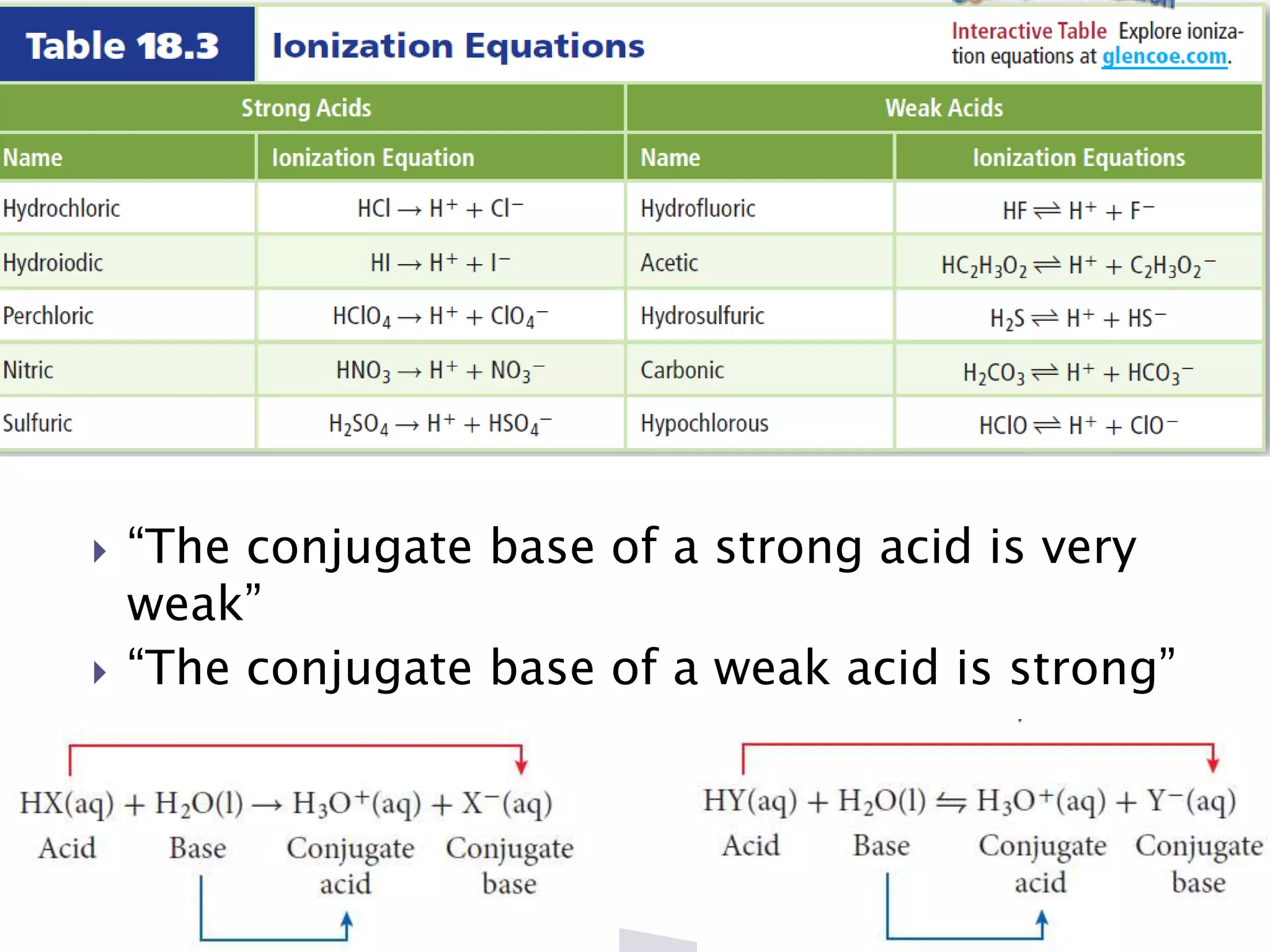
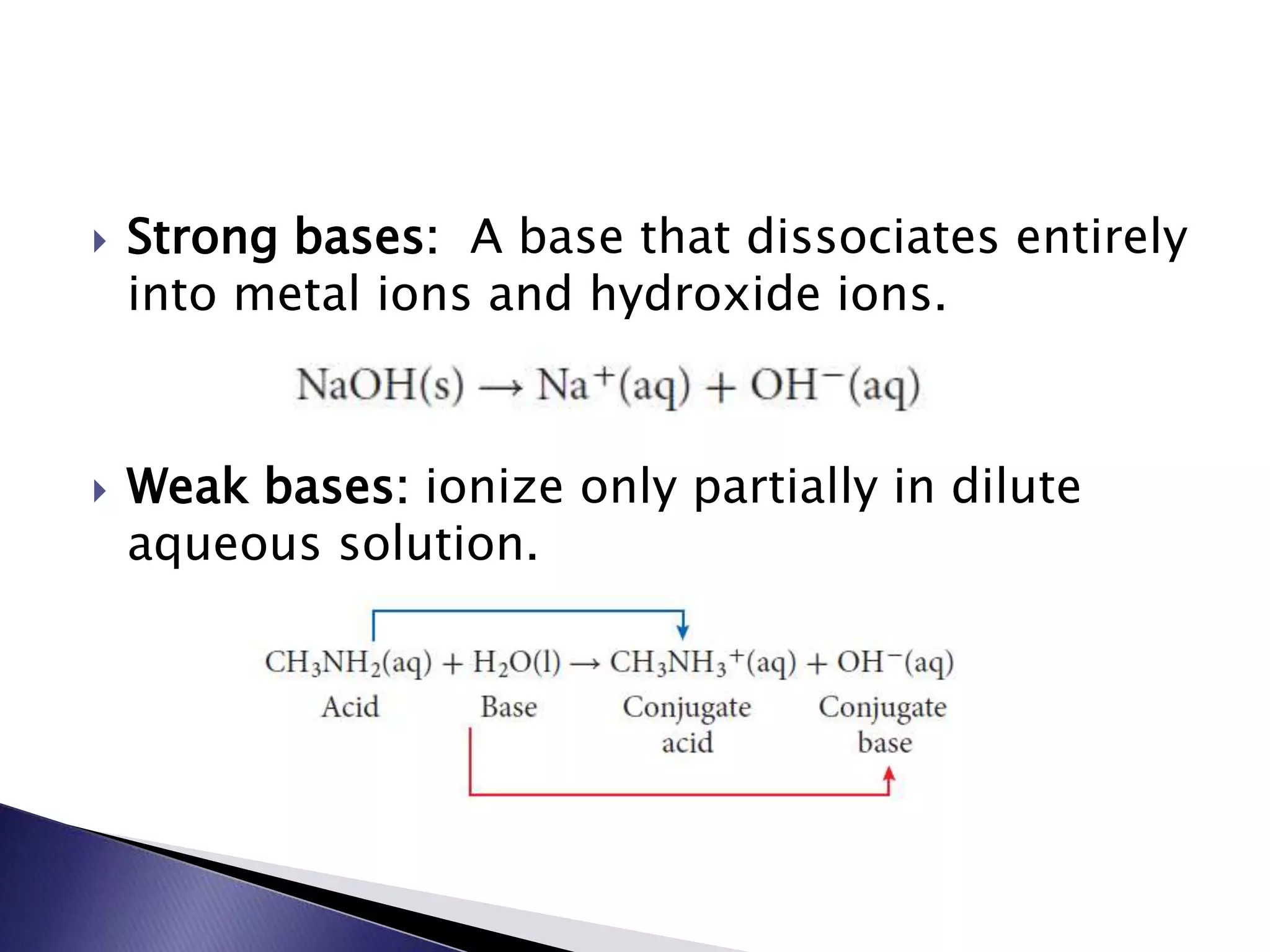
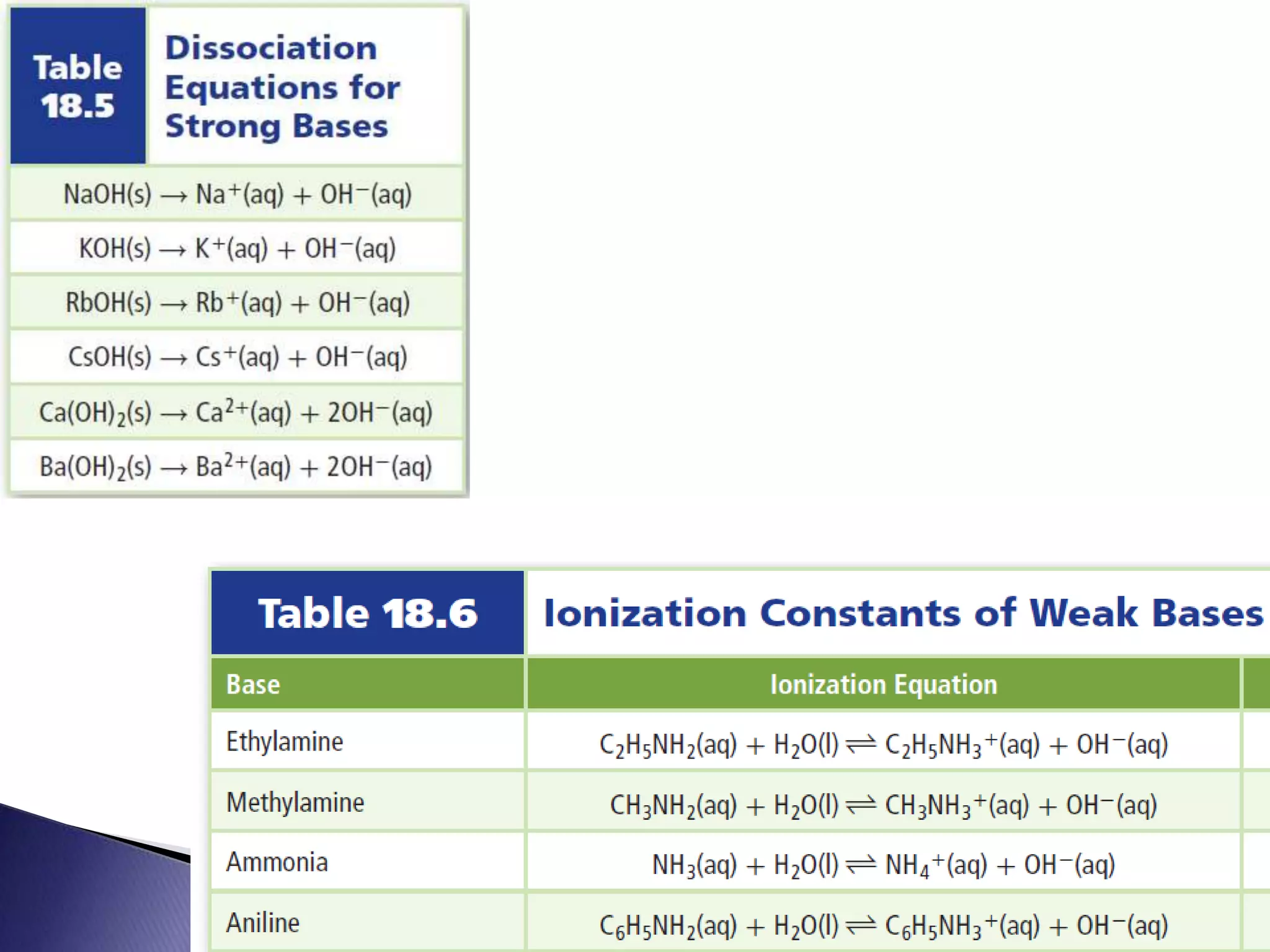
This document discusses acids and bases, including their properties and reactions. It defines acids as substances that release hydrogen ions in water and bases as those that release hydroxide ions. Acids and bases neutralize one another in a reaction that produces salt and water. The document also discusses acid-base theories, strong vs. weak acids/bases, pH, electrolytes, self-ionization of water, and acid-base indicators. Buffers are introduced as solutions that resist changes in pH when small amounts of acid or base are added.


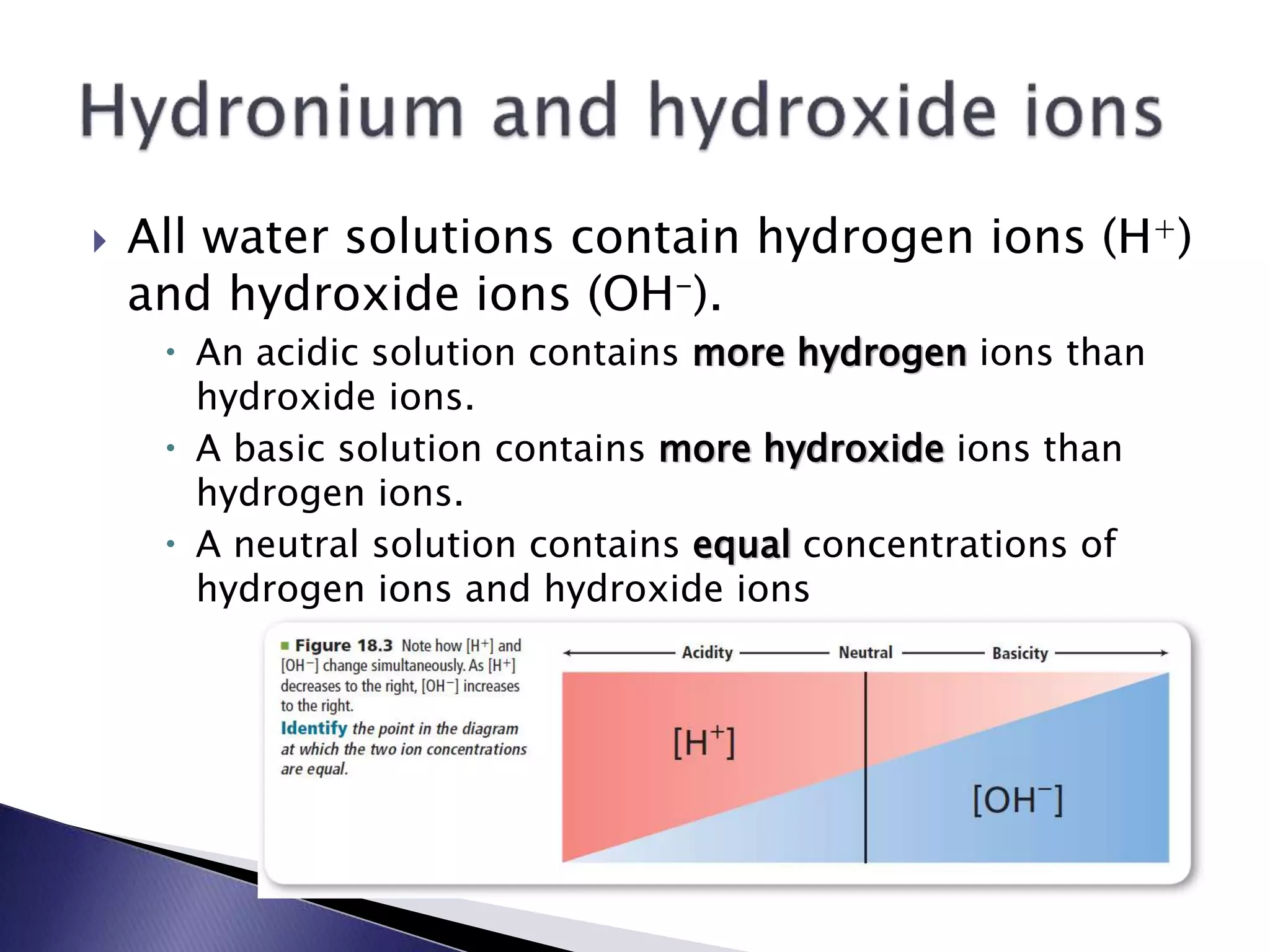





![ Pure water contains equal concentrations of H+ and OH- ions
produced by self-ionization.
The ion product constant for water is the value of the equilibrium
constant expression for the self-ionization of water.
In pure water at 25º C:
[H3O+] = 1.0 x 10-7 M
[OH-] = 1.0 x 10-7 M](https://image.slidesharecdn.com/unit3ale-140922233344-phpapp01/75/Unit-3-28-2048.jpg)
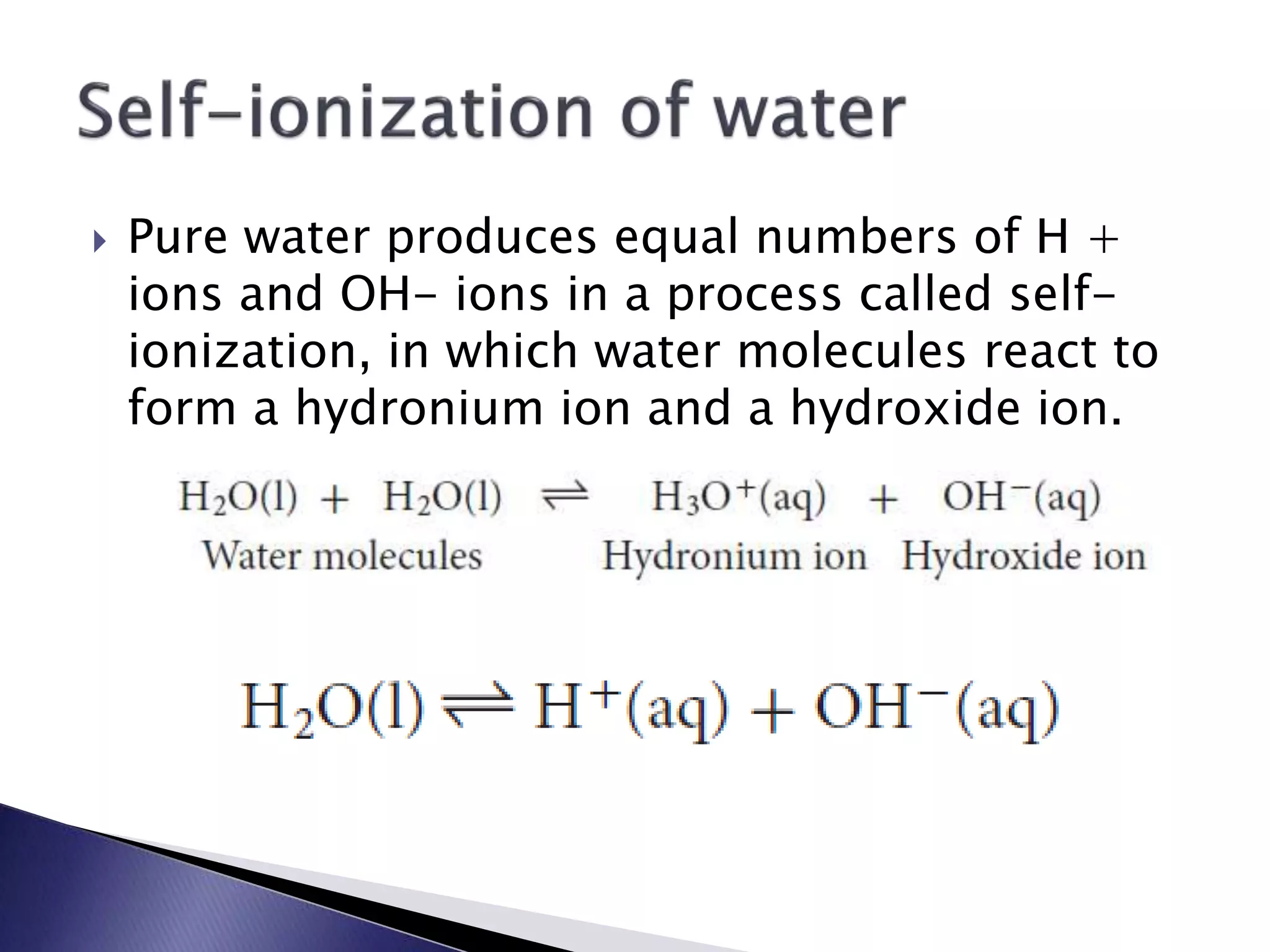
![ In pure water, the concentration of
hydronium ions equals the concentration of
hydroxide ions. This product is called the ion
product constant of water Kw
Acidic solutions • The [H+] is greater than 1.0 x 10-7 M
Basic solutions • The [H+] is lower than 1.0 x 10-7 M
• The [H+] is equal to 1.0 x 10-7 M
Neutral
solutions](https://image.slidesharecdn.com/unit3ale-140922233344-phpapp01/75/Unit-3-29-2048.jpg)