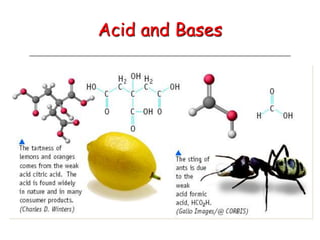
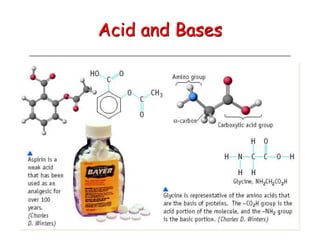
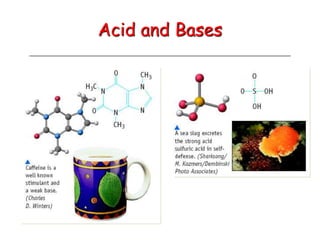
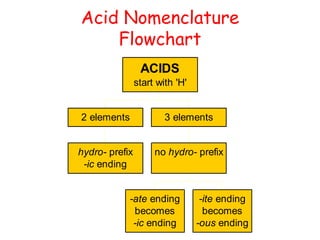
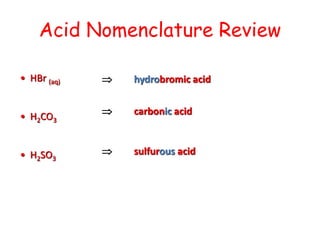
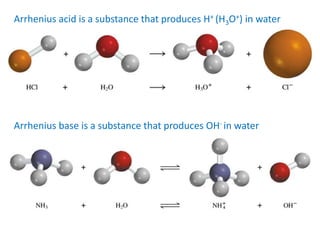
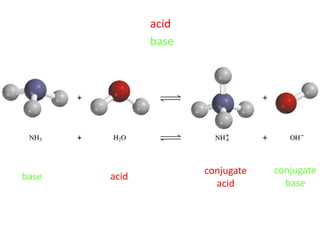
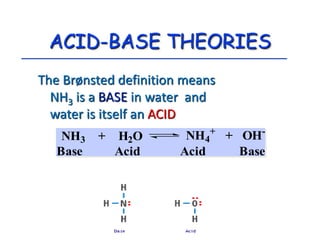
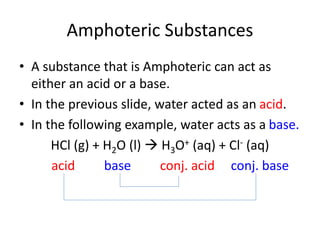
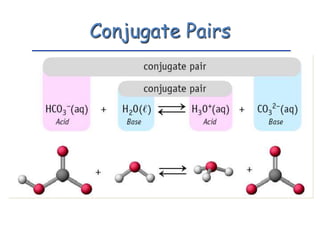
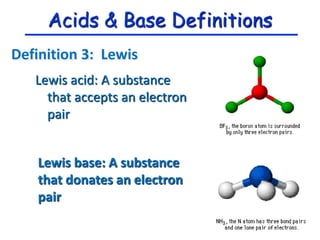
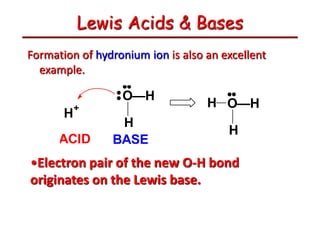
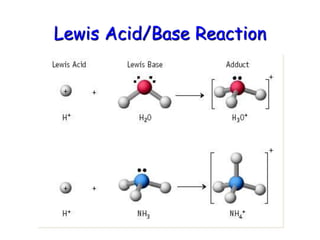
The document provides detailed information on acids and bases, including their properties, definitions, and nomenclature. It outlines different theories such as Arrhenius, Brønsted-Lowry, and Lewis definitions of acids and bases, and discusses the behavior of amphoteric substances. Additionally, it gives examples of common acids and bases, highlighting their chemical characteristics and reactions.