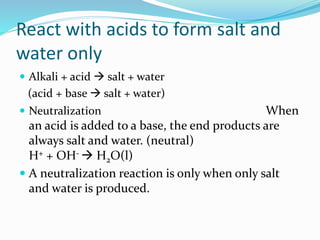
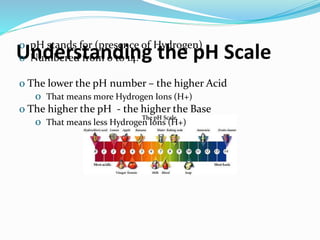
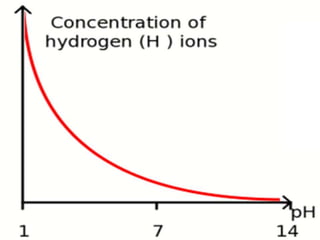
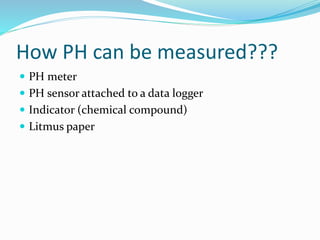
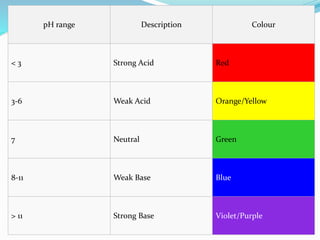
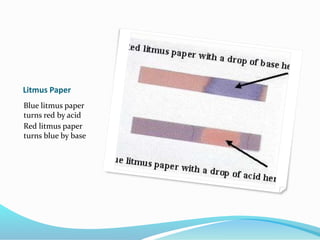
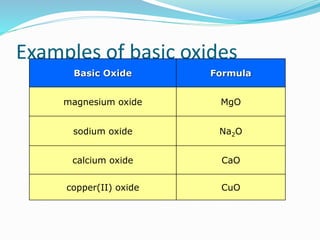
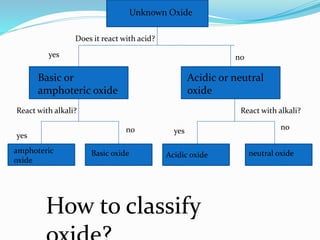
This document discusses acids and bases. It defines acids as substances that produce hydrogen ions in water, and bases as substances that produce hydroxide ions in water. Acids have a sour taste, turn litmus red, and react with metals and carbonates. Bases have a bitter taste, turn litmus blue, and react with acids to form salts and water. The document also discusses pH scale, uses of acids and bases, and how to classify different types of oxides such as acidic, basic, amphoteric, and neutral oxides based on their chemical properties.