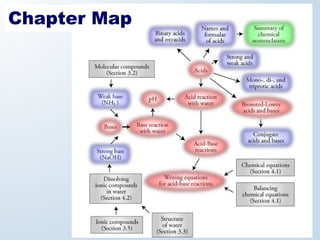
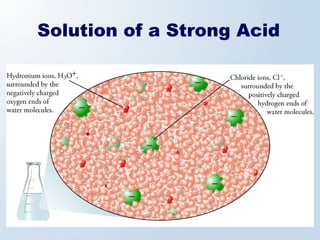
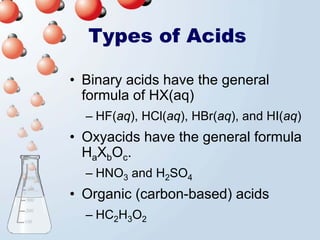
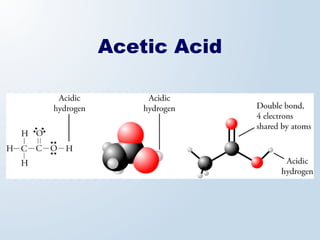
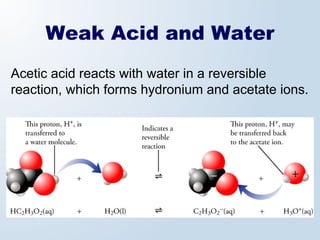
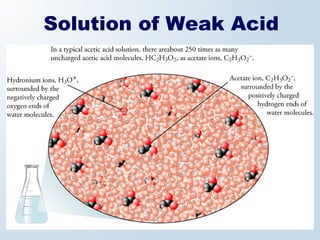
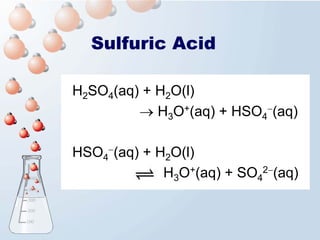
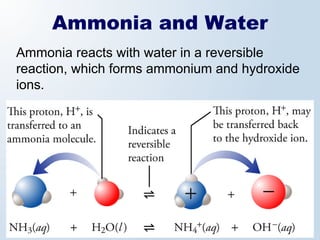
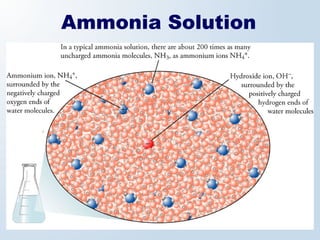
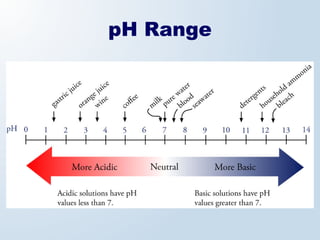
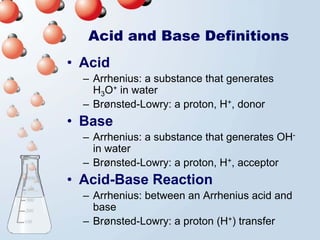
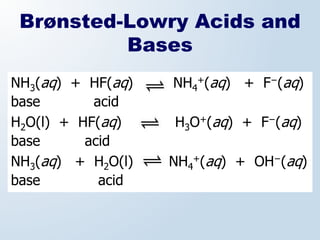
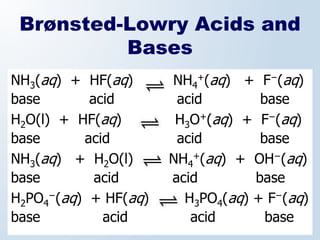
1. The document discusses acids and bases according to the Arrhenius and Brønsted-Lowry definitions. It describes the key characteristics of acids and bases and provides examples of strong and weak acids and bases.
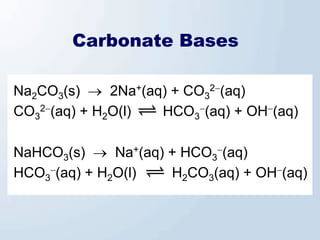
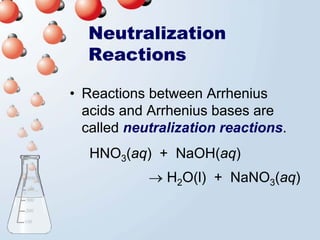
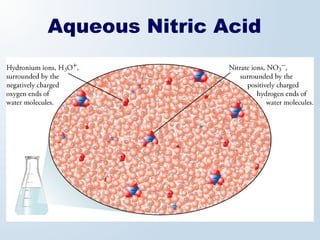
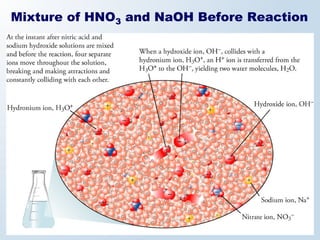
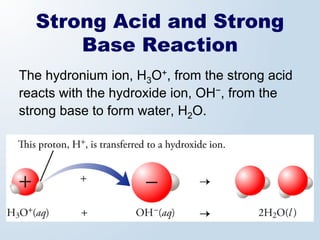
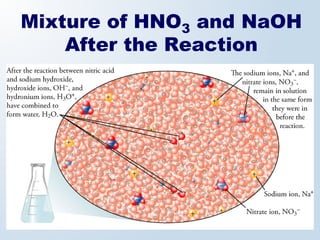
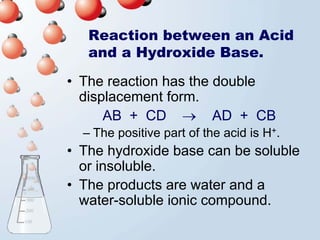
2. Neutralization reactions between acids and bases are discussed as double displacement reactions that produce water and a salt. Examples of reactions between acids and hydroxide or carbonate bases are provided.
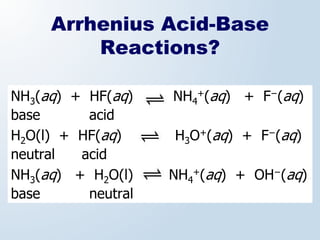
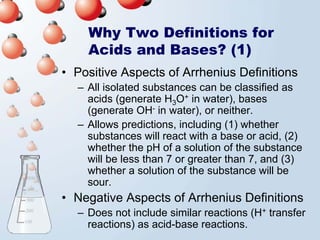
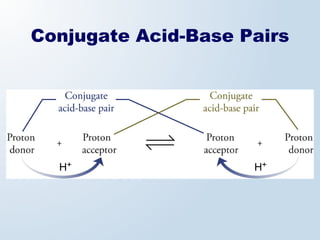
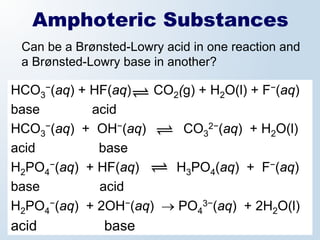
3. The document explains why both the Arrhenius and Brønsted-Lowry definitions are useful, noting their relative strengths and limitations. Key concepts like conjugate acid-base pairs and amphoteric substances are also introduced.